Electrochemistry studies the relationship between chemical reactions and electrical energy. It covers redox reactions, galvanic and electrolytic cells, electrode potentials, and the Nernst equation. Understanding electrochemical processes is crucial for topics like batteries, corrosion, and biological redox systems, which are vital for the MCAT.
Learning Objectives
In studying “Electrochemistry” for the MCAT, you should learn to identify the principles of redox reactions, including oxidation and reduction processes, and how electrons transfer between species. Understand the structure and function of electrochemical cells, such as galvanic (voltaic) and electrolytic cells, and analyze how cell potential relates to spontaneity and equilibrium. Evaluate the Nernst equation to determine the effect of concentration changes on cell voltage. Study the relationship between Gibbs free energy, standard electrode potentials, and equilibrium constants. Additionally, apply electrochemical principles to biological systems, such as membrane potentials and redox reactions in metabolism, ensuring you can solve MCAT-relevant problems related to energy production and electron flow.
Principles of Redox Reactions: Oxidation and Reduction Processes
Redox (reduction-oxidation) reactions are a fundamental concept in chemistry, involving the transfer of electrons between substances. These reactions are essential for many processes in biological systems, industrial applications, and environmental chemistry.
Basic Principles of Redox Reactions
1. Oxidation and Reduction:
- Oxidation refers to the loss of electrons by a molecule, atom, or ion.
- Reduction is the gain of electrons by a molecule, atom, or ion.
- In every redox reaction, one substance is oxidized (loses electrons), and another is reduced (gains electrons).
2. Oxidizing and Reducing Agents:
- An oxidizing agent is a substance that accepts electrons from another substance, thus undergoing reduction.
- A reducing agent is a substance that donates electrons to another substance, thus undergoing oxidation.
- The strength of an oxidizing or reducing agent depends on its ability to gain or lose electrons, respectively.
3. Electron Transfer:
- The core of redox chemistry is the transfer of electrons from one substance to another. This transfer of electrons can be direct (as in ionic substances) or through a series of chemical reactions.
4. Oxidation States:
- Oxidation states (or oxidation numbers) are used to keep track of electron transfers in redox reactions. They represent the total number of electrons that an atom either gains or loses compared to its elemental state.
- Changes in oxidation states during a reaction indicate which substances are oxidized and which are reduced.
Oxidizing and Reducing Agents
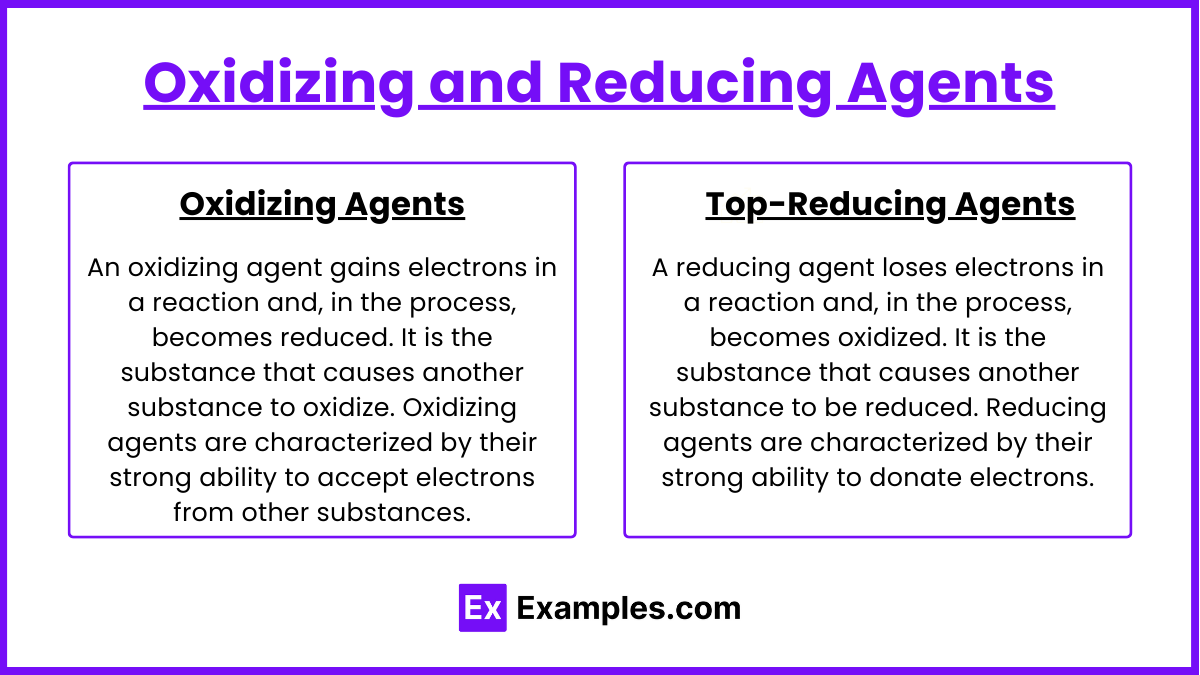
Oxidizing and reducing agents are substances that facilitate the transfer of electrons in redox (reduction-oxidation) reactions. These agents are crucial for driving chemical reactions by either gaining or losing electrons. Understanding their roles and characteristics helps in explaining various chemical processes, from industrial synthesis to biological metabolism.
Oxidizing Agents
An oxidizing agent gains electrons in a reaction and, in the process, becomes reduced. It is the substance that causes another substance to oxidize. Oxidizing agents are characterized by their strong ability to accept electrons from other substances.
Key Properties of Oxidizing Agents:
- They have a high affinity for electrons or have a high oxidation state that can be reduced through the gain of electrons.
- They often contain elements like oxygen, fluorine, or chlorine, which are highly electronegative.
Common Oxidizing Agents:
- Oxygen (O₂): Used in combustion reactions and in processes like corrosion.
- Chlorine (Cl₂): Used in water treatment and disinfectants.
- Potassium permanganate (KMnO₄): Used in organic synthesis and as a disinfectant.
- Hydrogen peroxide (H₂O₂): Used in bleaching, disinfection, and as a mild antiseptic.
- Nitric acid (HNO₃): Used in the production of fertilizers and in the manufacturing of explosives.
Reducing Agents
A reducing agent loses electrons in a reaction and, in the process, becomes oxidized. It is the substance that causes another substance to be reduced. Reducing agents are characterized by their strong ability to donate electrons.
Key Properties of Reducing Agents:
- They readily donate electrons, typically because they have low electronegativity or excess electrons.
- They can form stable byproducts after losing electrons, which is often essential for the viability of the redox reaction.
Common Reducing Agents:
- Hydrogen gas (H₂): Used to reduce metal oxides to metals.
- Carbon monoxide (CO): Used in metallurgy for reducing iron ore in the blast furnace to produce iron.
- Sodium borohydride (NaBH₄) and Lithium aluminum hydride (LiAlH₄): Commonly used in organic chemistry to reduce aldehydes, ketones, and esters to alcohols.
- Zinc (Zn) and Iron (Fe): Used in various industrial applications for reducing purposes.
- Electrons themselves: In electrochemical reactions, electrons move from the reducing agent at the anode to the oxidizing agent at the cathode.
Balancing Redox Reactions
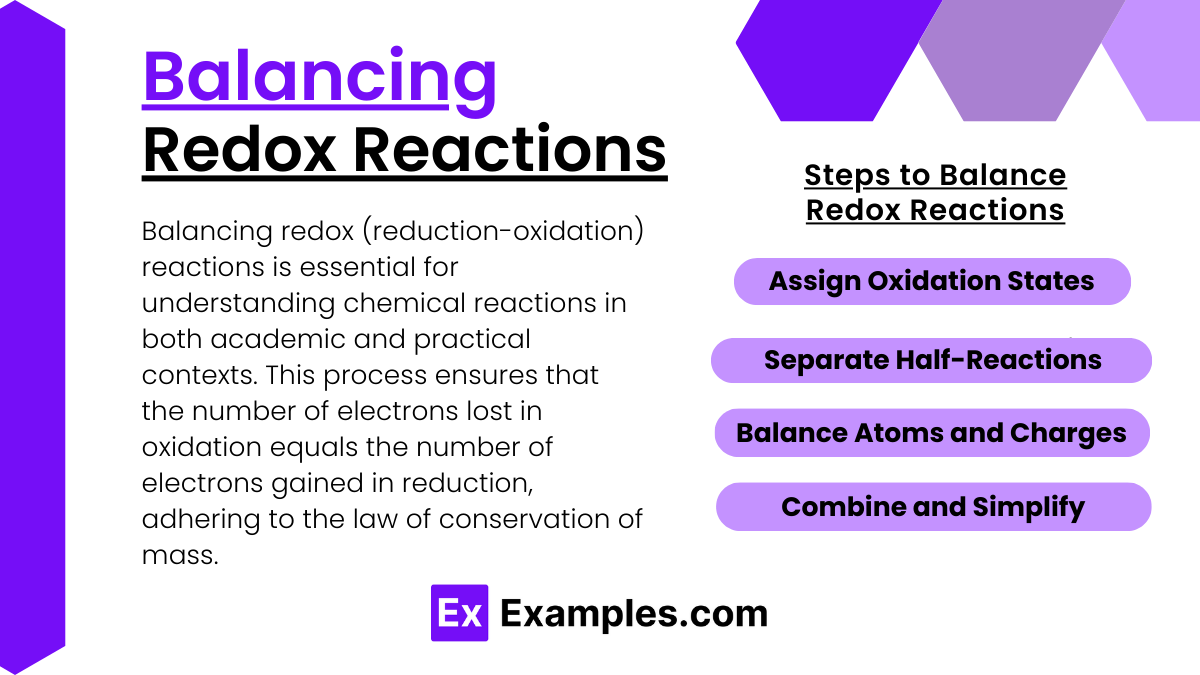
Balancing redox (reduction-oxidation) reactions is essential for understanding chemical reactions in both academic and practical contexts. This process ensures that the number of electrons lost in oxidation equals the number of electrons gained in reduction, adhering to the law of conservation of mass. Here’s a step-by-step guide on how to balance redox reactions using the half-reaction method, which is particularly useful for complex reactions.
Steps to Balance Redox Reactions
1. Write the Unbalanced Equation: Begin by writing down the skeletal equation of the reaction, showing the reactants and products without worrying about balancing them.
2. Identify Oxidation and Reduction Half-Reactions:
- Separate the overall reaction into two half-reactions—one for oxidation and one for reduction.
- Oxidation involves the loss of electrons, while reduction involves the gain of electrons.
3. Balance Atoms of Each Half-Reaction:
- First, balance all atoms except hydrogen and oxygen.
- Next, balance the oxygen atoms by adding water (H₂O) molecules.
- Balance the hydrogen atoms by adding hydrogen ions (H⁺) if the reaction occurs in acidic solution, or hydroxide ions (OH⁻) if it occurs in basic solution.
4. Balance the Electrons: Balance the charge in each half-reaction by adding electrons (e⁻). Add electrons to the left side of the reduction half-reaction (since it gains electrons) and to the right side of the oxidation half-reaction (since it loses electrons).
5. Equalize the Number of Electrons in Both Half-Reactions: Multiply the whole half-reactions by appropriate coefficients so that the number of electrons gained in the reduction half-reaction equals the number of electrons lost in the oxidation half-reaction.
6. Combine the Half-Reactions: Add the balanced half-reactions together. All electrons should cancel out, and you should be left with a balanced equation.
7. Check the Balance: Ensure that both the atoms and charges are balanced. The same number of each type of atom should appear on both sides of the equation, and the total charge should be the same on both sides.
Example: Balancing a Redox Reaction in Acidic Solution
Unbalanced Equation: MnO4− + Fe2+→Mn2+ + Fe3+
Step 1: Write Half-Reactions:
- Oxidation: Fe2+ → Fe3+ + e−
- Reduction: MnO4− + 5e−→ Mn2+
Step 2: Balance Atoms and Charge for Acidic Solution:
- Oxidation: Already balanced.
- Reduction: Balance oxygen by adding water: MnO4− + 5e− → Mn2+ + 8H+ (Added 4 water molecules to balance 4 oxygen atoms, balanced hydrogen by adding 8H+)
Step 3: Equalize and Combine:
- Multiply the oxidation half-reaction by 5 to balance the electrons: 5(Fe2+→Fe3++e−)
- Add the half-reactions: MnO4− + 5Fe2+ + 8H+ → Mn2+ + 5Fe3+ + 4H2O
Redox Reactions: Combined Oxidation and Reduction
Redox (reduction-oxidation) reactions are chemical processes where oxidation and reduction reactions occur simultaneously, involving the transfer of electrons between molecules. These fundamental reactions are pervasive in both nature and technology, underpinning processes from biological metabolism to energy storage.
Understanding Combined Oxidation and Reduction
Oxidation: Involves the loss of electrons. A species that loses electrons is oxidized. This species is referred to as the reducing agent because it donates electrons to another species.
Reduction: Involves the gain of electrons. A species that gains electrons is reduced. This species is referred to as the oxidizing agent because it accepts electrons from another species.
In any redox reaction, there cannot be oxidation without reduction and vice versa; they always occur together. The substance that gets oxidized transfers its electrons to the substance that gets reduced.
Basic Concepts
- Electron Transfer: The core of redox processes is the movement of electrons from one molecule (the reducer) to another (the oxidizer). This transfer changes the oxidation states of the reactants.
- Oxidation Numbers: To identify redox reactions and balance them, it’s essential to assign oxidation numbers to all atoms in the reactants and products. A change in these numbers indicates that oxidation and reduction have occurred.
- Use of a Half-Reaction Method: This approach splits the overall redox reaction into two separate half-reactions—one for oxidation and one for reduction. Each half-reaction is balanced separately for mass and charge, including electrons, which are then combined to form the balanced overall equation.
Examples
Example 1: Batteries in Everyday Devices
Batteries, such as those found in smartphones, laptops, and remote controls, are examples of electrochemical cells. These batteries convert stored chemical energy into electrical energy through spontaneous redox reactions. For instance, in a typical alkaline battery, zinc is oxidized at the anode while manganese dioxide is reduced at the cathode, creating a flow of electrons and powering devices.
Example 2: Electroplating
Electroplating is a process where a thin layer of metal is deposited onto the surface of another object using an electrolytic cell. For example, silver or gold plating involves immersing the object in a solution containing metal ions and passing an electric current through the solution. This causes metal ions to deposit onto the object, coating it with a layer of the desired metal. Electroplating is widely used for decorative purposes and in industries to prevent corrosion.
Example 3: Fuel Cells
Fuel cells are devices that generate electricity by converting the chemical energy of fuels, like hydrogen, directly into electrical energy through electrochemical reactions. In a hydrogen fuel cell, hydrogen is oxidized at the anode while oxygen is reduced at the cathode, producing water as a byproduct and generating a continuous flow of electrical energy. Fuel cells are used in various applications, including powering electric vehicles and backup power systems.
Example 4: Corrosion
Corrosion is an electrochemical process in which metals deteriorate due to reactions with their environment, such as the rusting of iron. When iron is exposed to moisture and oxygen, it undergoes oxidation, losing electrons to form iron oxide (rust). This process involves both anodic and cathodic reactions, making corrosion a common electrochemical phenomenon that affects the durability of structures and machinery.
Example 5: Electrolysis of Water
Electrolysis is the process of using electrical energy to drive a non-spontaneous chemical reaction. In the electrolysis of water, an electric current is passed through water, causing it to break down into hydrogen and oxygen gases. Hydrogen is produced at the cathode, while oxygen is generated at the anode. This process is an important method for producing hydrogen fuel and is used in various industrial applications.
Practice Questions
Question 1
What is the primary function of a galvanic cell?
A) To convert chemical energy into thermal energy
B) To convert chemical energy into electrical energy
C) To convert electrical energy into chemical energy
D) To store energy indefinitely
Correct Answer: B) To convert chemical energy into electrical energy.
Explanation: A galvanic cell, also known as a voltaic cell, generates electrical energy from spontaneous chemical reactions. It does this through oxidation and reduction reactions occurring in separate half-cells, where chemical energy is transformed into electrical energy. Option A is incorrect because galvanic cells do not primarily produce thermal energy. Option C describes the function of an electrolytic cell, and option D is misleading, as galvanic cells do not store energy indefinitely; they produce energy as long as reactants are available.
Question 2
Which of the following statements about an electrolytic cell is true?
A) It generates electrical energy from spontaneous reactions.
B) It requires an external voltage source to drive the reaction.
C) It operates without any electrodes.
D) It is used to measure the voltage of a chemical reaction.
Correct Answer: B) It requires an external voltage source to drive the reaction.
Explanation: An electrolytic cell requires an external voltage source to drive non-spontaneous reactions. This is the main characteristic that differentiates it from a galvanic cell, which generates electrical energy from spontaneous reactions. Option A is incorrect because electrolytic cells do not generate energy; they consume energy. Option C is false, as electrolytic cells do have electrodes that facilitate the reactions, and option D is misleading, as electrolytic cells do not measure voltage; they perform electrolysis.
Question 3
In an electrochemical reaction, what occurs at the anode?
A) Reduction of species takes place.
B) Oxidation of species takes place.
C) No reaction occurs.
D) The voltage is measured.
Correct Answer: B) Oxidation of species takes place.
Explanation: In an electrochemical reaction, the anode is the electrode where oxidation occurs, meaning that a species loses electrons. In a galvanic cell, this process releases energy, while in an electrolytic cell, it requires an external power source. Option A is incorrect, as reduction occurs at the cathode, not the anode. Option C is false because a reaction takes place at the anode, and option D is misleading since voltage measurement is a separate function that does not specifically relate to the processes occurring at the anode.