In AP Biology, understanding the makeup and properties of macromolecules is essential as they form the basis of life. Macromolecules like carbohydrates, lipids, proteins, and nucleic acids interact with water and water vapor, influencing their chemical properties and biological functions. These properties, such as solubility, reactivity, and structural integrity, are crucial for processes like energy storage, cellular structure, and genetic information transmission, highlighting their significance in biological systems.
Learning Objectives
By studying the makeup and properties of macromolecules in AP Biology, students will learn about the structure and functions of carbohydrates, lipids, proteins, and nucleic acids. They will understand how these macromolecules interact with water, their roles in biological processes, and their chemical properties such as solubility, reactivity, and structural integrity. Additionally, students will explore the importance of these macromolecules in energy storage, cellular structure, and genetic information transmission, providing a comprehensive understanding of their significance in living organisms.
Types of Macromolecules
Carbohydrates
Structure
Monomers: Monosaccharides (simple sugars like glucose, fructose)
Polymers: Disaccharides (sucrose, lactose) and polysaccharides (starch, glycogen, cellulose)
Properties
Energy Storage: Starch (plants), glycogen (animals)
Structural Components: Cellulose (plant cell walls), chitin (exoskeletons of arthropods)
Solubility: Generally soluble in water due to hydroxyl groups, forming hydrogen bonds with water molecules
Functions
Energy Source: Quick energy via glucose
Structural Support: Cellulose in plants, chitin in arthropods
Cell Recognition: Glycoproteins and glycolipids on cell surfaces
Lipids
Structure
Components: Glycerol and fatty acids (triglycerides), phosphate groups (phospholipids), four-ring structures (steroids)
Types: Triglycerides, phospholipids, steroids, and waxes
Properties
Hydrophobic: Insoluble in water due to nonpolar hydrocarbon chains
Saturated vs. Unsaturated: Saturated fats have no double bonds (solid at room temperature), unsaturated fats have one or more double bonds (liquid at room temperature)
Functions
Energy Storage: Long-term energy storage in adipose tissue
Membrane Structure: Phospholipids form bilayers in cell membranes
Hormones: Steroids like testosterone and estrogen
Insulation and Protection: Fat provides insulation and cushioning
Proteins
Structure
Monomers: Amino acids (20 different kinds)
Polymers: Polypeptides (proteins are made up of one or more polypeptides)
Levels of Protein Structure
Primary Structure: Sequence of amino acids
Secondary Structure: Alpha helices and beta sheets formed by hydrogen bonding
Tertiary Structure: 3D folding due to interactions between R groups (side chains)
Quaternary Structure: Association of multiple polypeptide chains
Properties
Diverse Functions: Enzymes, structural proteins, transport proteins, antibodies
Solubility: Depends on the amino acid composition; some proteins are soluble in water, others are not
Functions
Catalysis: Enzymes accelerate biochemical reactions
Structure: Collagen in connective tissues, keratin in hair and nails
Transport: Hemoglobin carries oxygen in blood
Defense: Antibodies in the immune system
Regulation: Hormones like insulin
Nucleic Acids
Structure
Monomers: Nucleotides (composed of a sugar, phosphate group, and nitrogenous base)
Polymers: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid)
Properties
Double Helix: DNA is double-stranded and forms a double helix
Single-Stranded: RNA is usually single-stranded
Base Pairing: DNA (A-T, G-C), RNA (A-U, G-C)
Functions
Genetic Information: DNA stores genetic information
Protein Synthesis: RNA is involved in translating genetic information into proteins (mRNA, tRNA, rRNA)
Regulation: Some RNA molecules regulate gene expression
Polymerization and Breakdown of Macromolecules
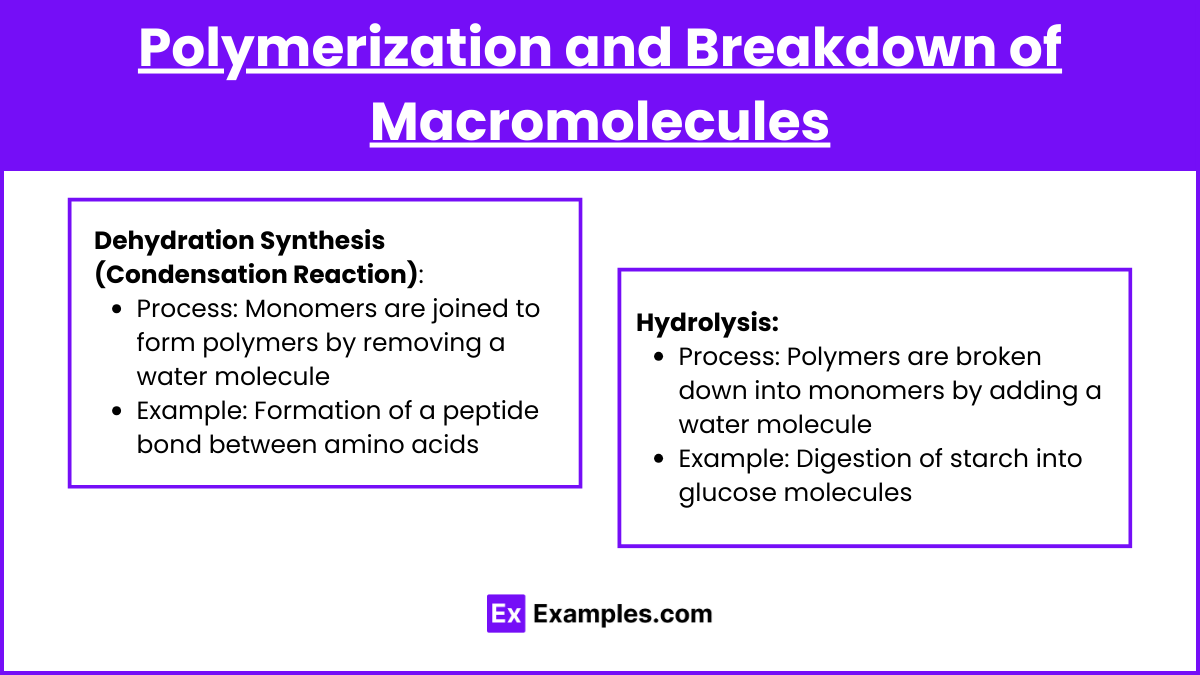
Dehydration Synthesis (Condensation Reaction)
Process: Monomers are joined to form polymers by removing a water molecule
Example: Formation of a peptide bond between amino acids
Hydrolysis
Process: Polymers are broken down into monomers by adding a water molecule
Example: Digestion of starch into glucose molecules
Enzymes and Macromolecules
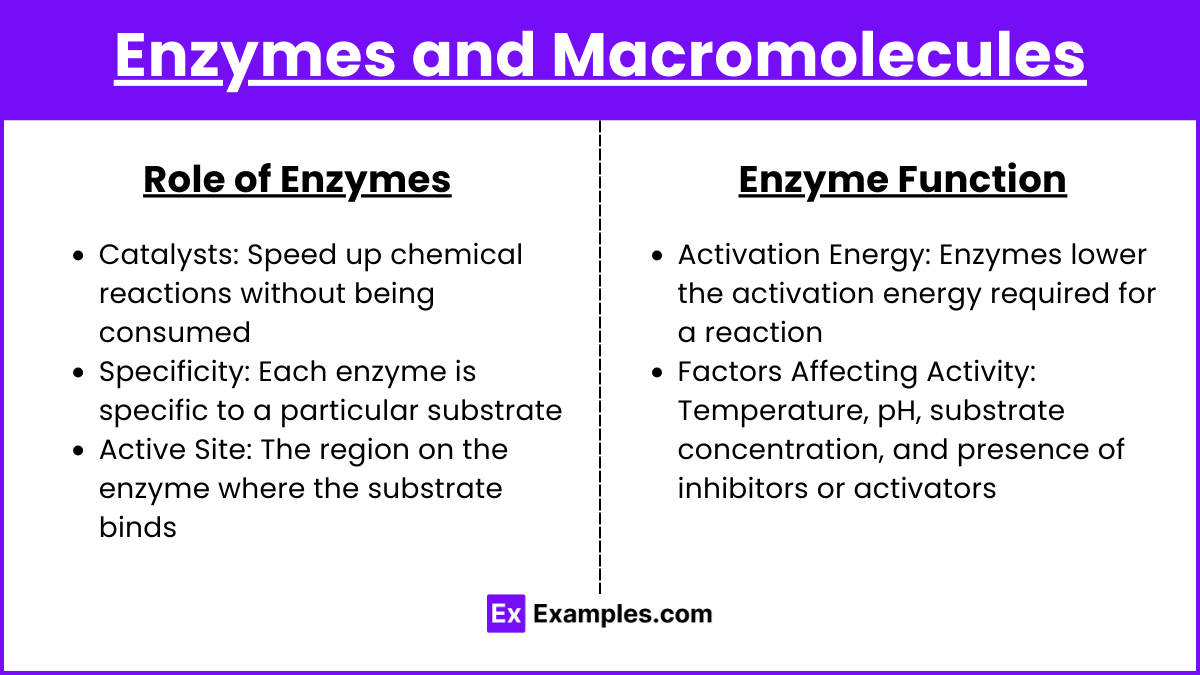
Role of Enzymes
Catalysts: Speed up chemical reactions without being consumed
Specificity: Each enzyme is specific to a particular substrate
Active Site: The region on the enzyme where the substrate binds
Enzyme Function
Activation Energy: Enzymes lower the activation energy required for a reaction
Factors Affecting Activity: Temperature, pH, substrate concentration, and presence of inhibitors or activators
Examples of Macromolecules in Biological Systems
Carbohydrates
Glycogen: Energy storage in liver and muscle cells
Cellulose: Provides structural support in plant cell walls
Sucrose: Transported in plant sap
Lipids
Triglycerides: Stored in adipose tissue as energy reserves
Phospholipids: Form the lipid bilayer of cell membranes
Cholesterol: A component of cell membranes and precursor of steroid hormones
Proteins
Hemoglobin: Transports oxygen in red blood cells
Antibodies: Immune proteins that recognize and neutralize pathogens
Enzymes: Catalysts like amylase, lipase, and protease
Nucleic Acids
DNA: Stores genetic information in the nucleus
mRNA: Carries genetic information from DNA to the ribosome for protein synthesis
tRNA: Transfers amino acids to the ribosome during protein synthesis


