In AP Biology, the study of enzymes is crucial for understanding cellular processes. Enzymes are complex compounds that catalyze biochemical reactions in the cytosol and other cell compartments. They are essential for breaking down substances like cellulose in plant cells, facilitating metabolic activities. Enzymes’ specificity and efficiency are vital for maintaining life, making their structure and function key topics in advanced biology.
Learning Objectives
Learning objectives for AP Biology on the structure and function of enzymes include understanding how enzymes break down polysaccharides like cellulose in the cell wall, and how they operate in organelles like chloroplasts and peroxisomes. Students should explain the role of ribosomes in enzyme production, describe specific enzymes like nitrogenase and catalase, and understand their importance in metabolic processes. Mastery of these concepts is essential for a comprehensive understanding of enzyme function in biological systems.
Enzyme Structure
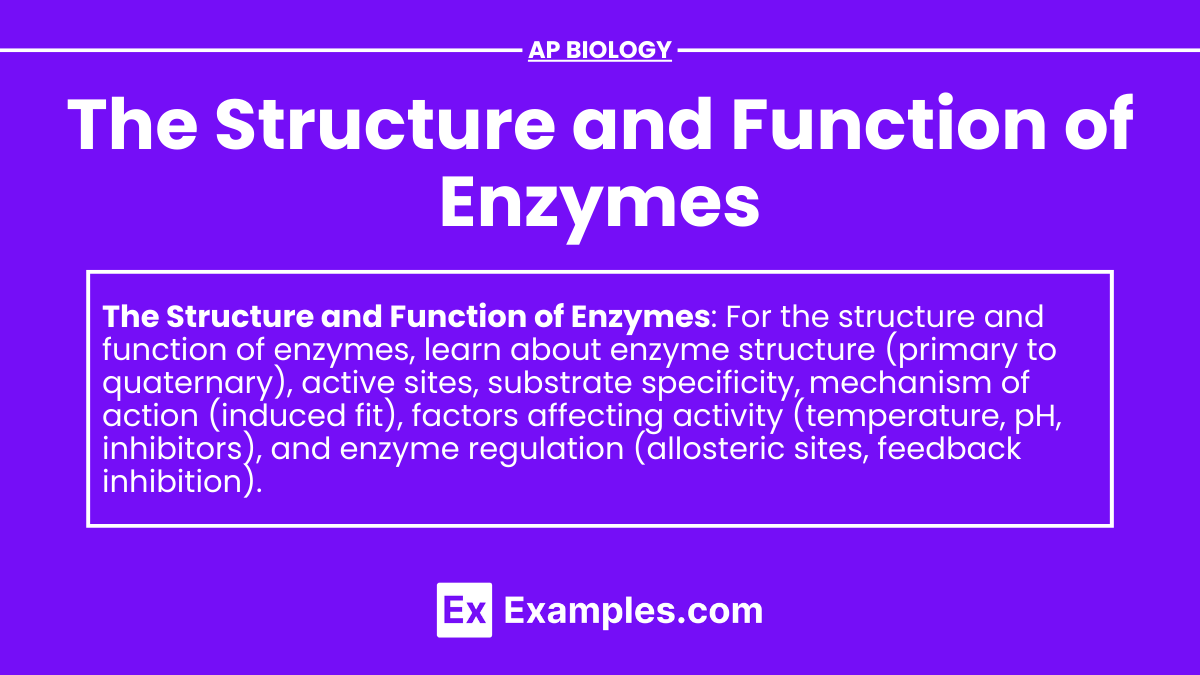
Primary Structure
- Amino Acid Sequence: Enzymes are proteins made up of long chains of amino acids. The sequence of these amino acids determines the enzyme’s properties and function.
Secondary Structure
- Alpha Helices and Beta Sheets: The amino acid chain folds into alpha helices and beta sheets due to hydrogen bonding.
Tertiary Structure
- Three-Dimensional Shape: The overall 3D shape of an enzyme is formed by further folding and interactions, such as disulfide bridges, hydrophobic interactions, and ionic bonds. This shape is critical for the enzyme’s functionality.
Quaternary Structure
- Subunit Assembly: Some enzymes consist of multiple protein subunits, which come together to form the active enzyme.
Active Site and Specificity
- Active Site: The region of the enzyme where the substrate binds. It is specifically shaped to fit the substrate, ensuring high specificity.
- Enzyme-Substrate Complex: When a substrate binds to the enzyme’s active site, it forms an enzyme-substrate complex, facilitating the chemical reaction.
Mechanism of Enzyme Action
Lowering Activation Energy
- Enzymes lower the activation energy required for a reaction, enabling the reaction to proceed more quickly.
Induced Fit Model
- The enzyme changes shape slightly to accommodate the substrate, enhancing the reaction’s efficiency.
Catalytic Cycle
- Binding: The substrate binds to the enzyme’s active site.
- Reaction: The enzyme catalyzes the conversion of the substrate into products.
- Release: The products are released, and the enzyme is free to catalyze another reaction.
Factors Affecting Enzyme Activity
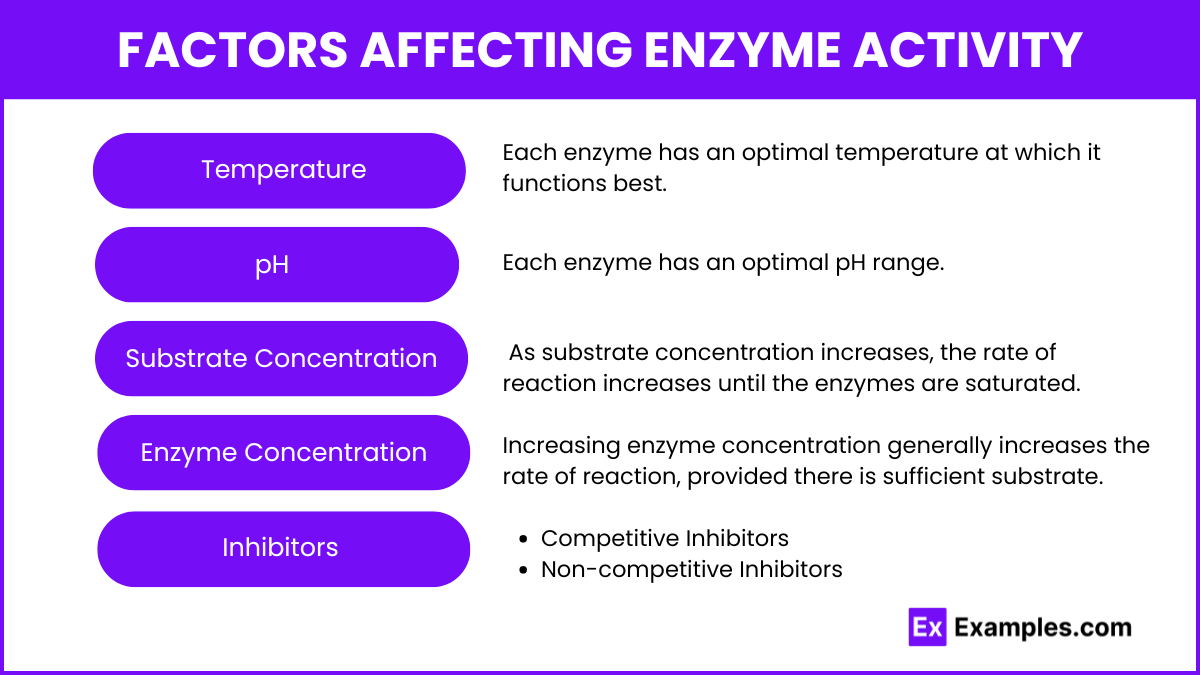
Temperature
- Optimum Temperature: Each enzyme has an optimal temperature at which it functions best.
- Denaturation: Extreme temperatures can denature enzymes, altering their structure and function.
pH
- Optimum pH: Each enzyme has an optimal pH range.
- Denaturation: Deviations from this range can denature the enzyme.
Substrate Concentration
- Saturation: As substrate concentration increases, the rate of reaction increases until the enzymes are saturated.
Enzyme Concentration
- Increasing enzyme concentration generally increases the rate of reaction, provided there is sufficient substrate.
Inhibitors
- Competitive Inhibitors: Compete with the substrate for the active site.
- Non-competitive Inhibitors: Bind to a different part of the enzyme, altering its function.
Types of Enzymes
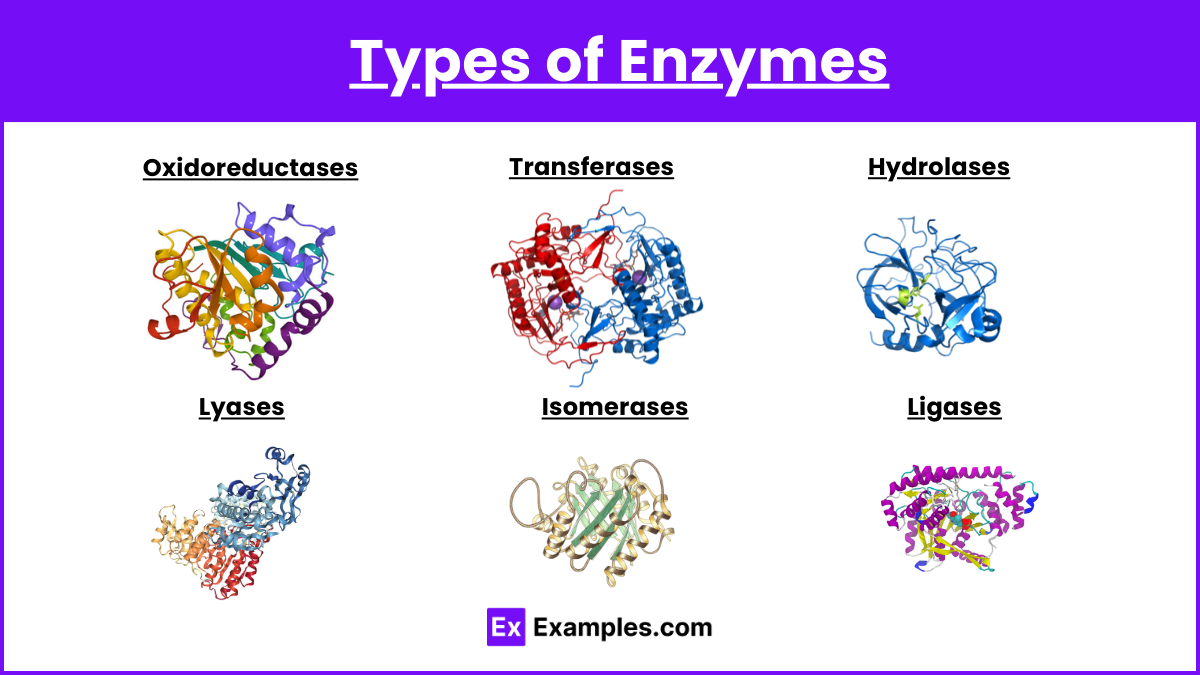
Oxidoreductases
- Catalyze oxidation-reduction reactions.
Transferases
- Transfer functional groups between molecules.
Hydrolases
- Catalyze the hydrolysis of various bonds.
Lyases
- Break bonds without hydrolysis or oxidation.
Isomerases
- Catalyze isomerization changes within a single molecule.
Ligases
- Catalyze the joining of two molecules with the input of energy (usually ATP).
Coenzymes and Cofactors
- Coenzymes: Organic molecules that assist enzymes, often derived from vitamins.
- Cofactors: Inorganic ions that assist enzyme activity.
Examples of Enzymes and Their Functions
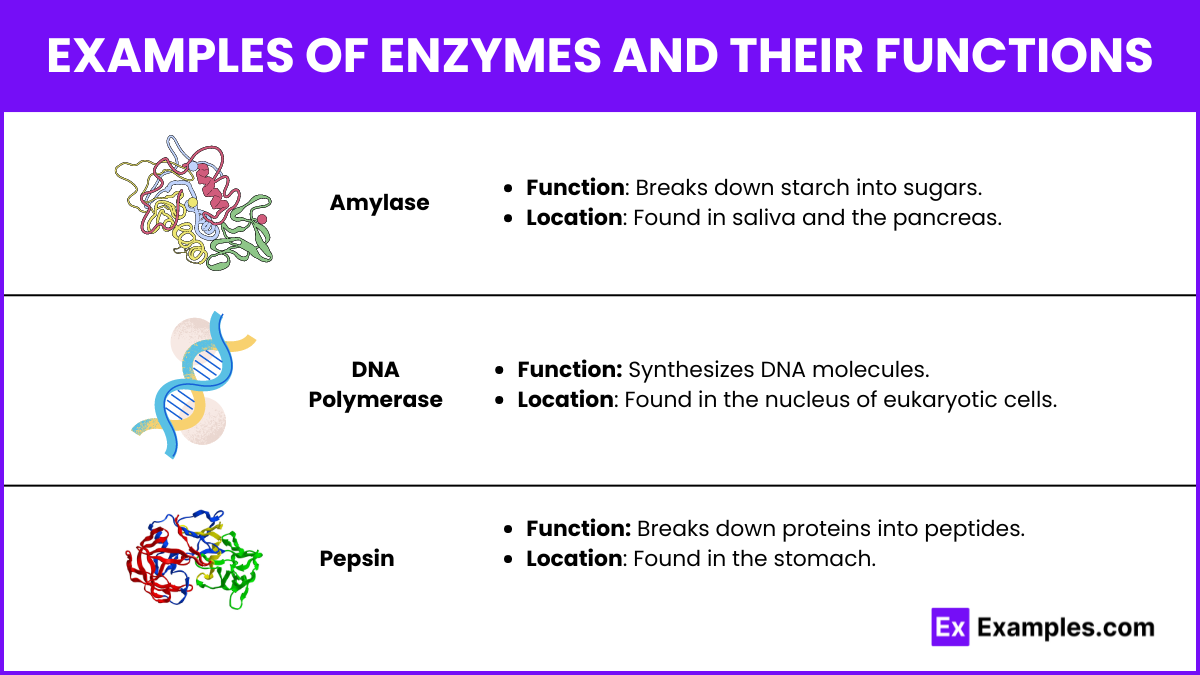
Amylase
- Function: Breaks down starch into sugars.
- Location: Found in saliva and the pancreas.
DNA Polymerase
- Function: Synthesizes DNA molecules.
- Location: Found in the nucleus of eukaryotic cells.
Pepsin
- Function: Breaks down proteins into peptides.
- Location: Found in the stomach.
Enzyme Regulation
Allosteric Regulation
- Enzyme activity is regulated by the binding of molecules at sites other than the active site, causing a conformational change.
Feedback Inhibition
- The end product of a metabolic pathway inhibits an enzyme involved earlier in the pathway, regulating the pathway’s activity.
Post-translational Modifications
- Enzymes can be activated or deactivated through modifications such as phosphorylation or glycosylation.