Learning Objectives
In studying Acid-Base Reactions and Buffers for the AP Chemistry exam, you should aim to understand the different theories of acids and bases (Arrhenius, Brønsted-Lowry, and Lewis), distinguish between strong and weak acids and bases, and identify conjugate acid-base pairs. You should be able to perform calculations involving pH, pOH, and the use of the Henderson-Hasselbalch equation. Additionally, you should grasp the concept of buffer solutions, their components, and how they resist pH changes. Finally, you should be able to interpret titration curves, determine equivalence points, and select appropriate indicators for titrations. Mastery of these objectives will help you solve complex problems and apply these concepts in various chemical contexts.
Free AP Chemistry Practice Test
Introduction
Acid-base reactions and buffers are fundamental concepts in chemistry, essential for understanding a wide range of chemical processes. Acids and bases interact in various ways, producing salts and water in neutralization reactions. The strength of acids and bases, as well as their ability to donate or accept protons, defines their behavior in solutions. Buffers, on the other hand, are crucial for maintaining pH stability in biological and chemical systems by neutralizing small amounts of added acid or base.
Acid-Base Reactions
What are Acid-Base Reactions?

Acid-base reactions involve the transfer of protons (H⁺ ions) between reactants. In these reactions, an acid donates protons, while a base accepts protons. The result is the formation of a salt and often water. These reactions are key to understanding many chemical processes and are classified by different theories such as Arrhenius, Brønsted-Lowry, and Lewis, each providing unique perspectives on acid and base behavior.
Acid-Base Theories
1. Arrhenius Theory
Acid: Produces H⁺ ions in aqueous solution.
Example: HCl → H⁺ + Cl⁻
Base: Produces OH⁻ ions in aqueous solution.
Example: NaOH → Na⁺ + OH⁻
2. Brønsted-Lowry Theory
Acid: Proton (H⁺ ion) donor.
Example: NH₄⁺ → NH₃ + H⁺
Base: Proton (H⁺ ion) acceptor.
Example: NH₃ + H⁺ → NH₄⁺
3. Lewis Theory
Acid: Electron pair acceptor.
Example: BF₃ + F⁻ → BF₄⁻
Base: Electron pair donor.
Example: NH₃ + BF₃ → NH₃ - BF₃
Strong vs. Weak Acids and Bases
Characteristic | Strong Acids | Weak Acids | Strong Bases | Weak Bases |
|---|---|---|---|---|
Definition | Completely ionize in solution | Partially ionize in solution | Completely dissociate in solution | Partially dissociate in solution |
Ionization/Dissociation | Nearly 100% ionization | Less than 100% ionization | Nearly 100% dissociation | Less than 100% dissociation |
Examples | HCl, H₂SO₄, HNO₃ | CH₃COOH, HF, HCN | NaOH, KOH, Ba(OH)₂ | NH₃, CH₃NH₂, C₆H₅NH₂ (aniline) |
pH Level in Solution | Very low pH (e.g., 1-2) | Higher pH compared to strong acids (e.g., 3-6) | Very high pH (e.g., 13-14) | Lower pH compared to strong bases (e.g., 8-11) |
Conductivity | High (strong electrolytes) | Moderate (weak electrolytes) | High (strong electrolytes) | Moderate (weak electrolytes) |
Acid/Base Ionization Constant (Ka/Kb) | Large Kₐ (small pKₐ) | Small Kₐ (large pKₐ) | Large K₆ (small pK₆) | Small K₆ (large pK₆) |
Acid-Base Reactions
Neutralization Reactions
Neutralization occurs when an acid reacts with a base to produce a salt and water.
Example: HCl + NaOH → NaCl + H₂O
Hydrolysis Reactions
Hydrolysis involves the reaction of a salt with water to form an acidic or basic solution.
Example: NH₄Cl + H₂O → NH₄OH + HCl
Conjugate Acid-Base Pairs
Each acid has a conjugate base, and each base has a conjugate acid. This concept is key to understanding the Brønsted-Lowry theory of acids and bases.
Example:
NH₄⁺ (conjugate acid of NH₃)
NH₃ (conjugate base of NH₄⁺)
Amphoteric Substances
Amphoteric substances can act as both acids and bases.
Example: H₂O + H⁺ → H₃O⁺
Buffers
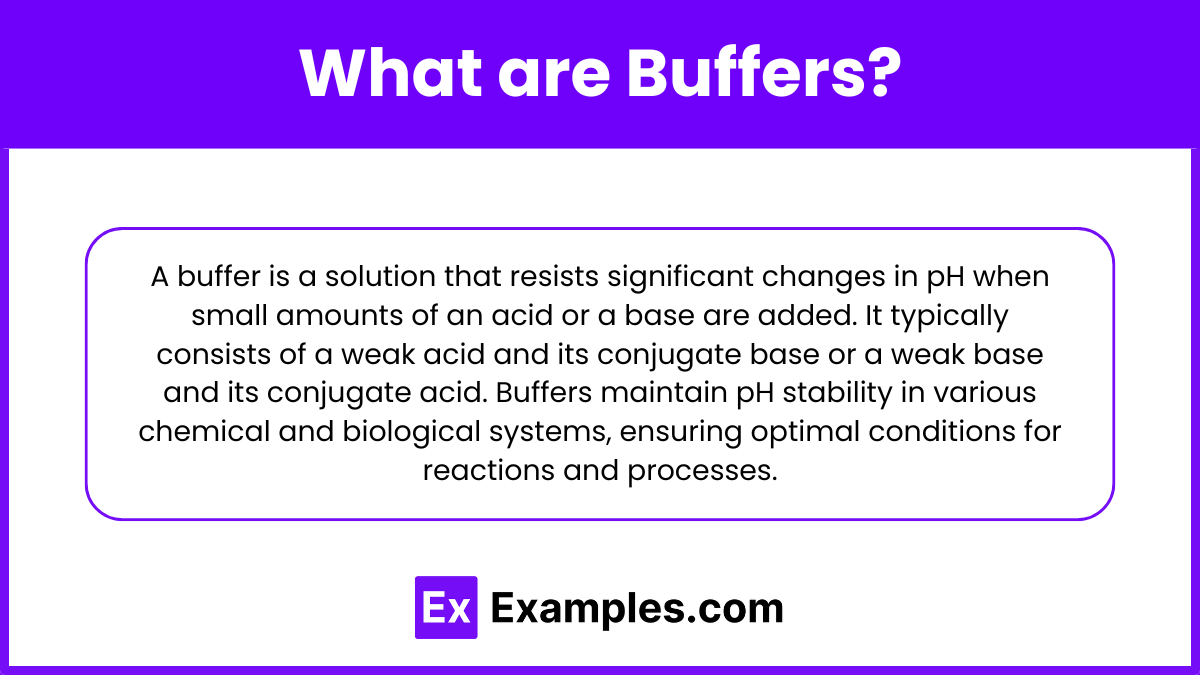
What are Buffers?
A buffer is a solution that resists significant changes in pH when small amounts of an acid or a base are added. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers maintain pH stability in various chemical and biological systems, ensuring optimal conditions for reactions and processes.
Components of a Buffer
A buffer solution is composed of two key components:
Weak Acid and Its Conjugate Base:
Weak Acid: Partially ionizes in solution to release H⁺ ions.
Conjugate Base: The ion or molecule that remains after the weak acid has donated a proton.
Example: Acetic acid CH₃COOH and sodium acetate CH₃COONa.
Weak Base and Its Conjugate Acid:
Weak Base: Partially accepts H⁺ ions in solution.
Conjugate Acid: The ion or molecule that forms when the weak base accepts a proton.
Example: Ammonia NH₃ and ammonium chloride NH₄Cl.
Buffer Action
Buffers maintain the pH of a solution by neutralizing added acids or bases. This stability is achieved through the equilibrium between the weak acid and its conjugate base, or the weak base and its conjugate acid. Here's how buffer action works:
Addition of Acid (H⁺)
When an acid (H⁺) is added to the buffer, the conjugate base component of the buffer reacts with the H⁺ ions to form the weak acid, thereby minimizing the change in pH.
Addition of Base (OH⁻)
When a base (OH⁻) is added to the buffer, the weak acid component of the buffer reacts with the OH⁻ ions to form water and the conjugate base, thereby minimizing the change in pH.
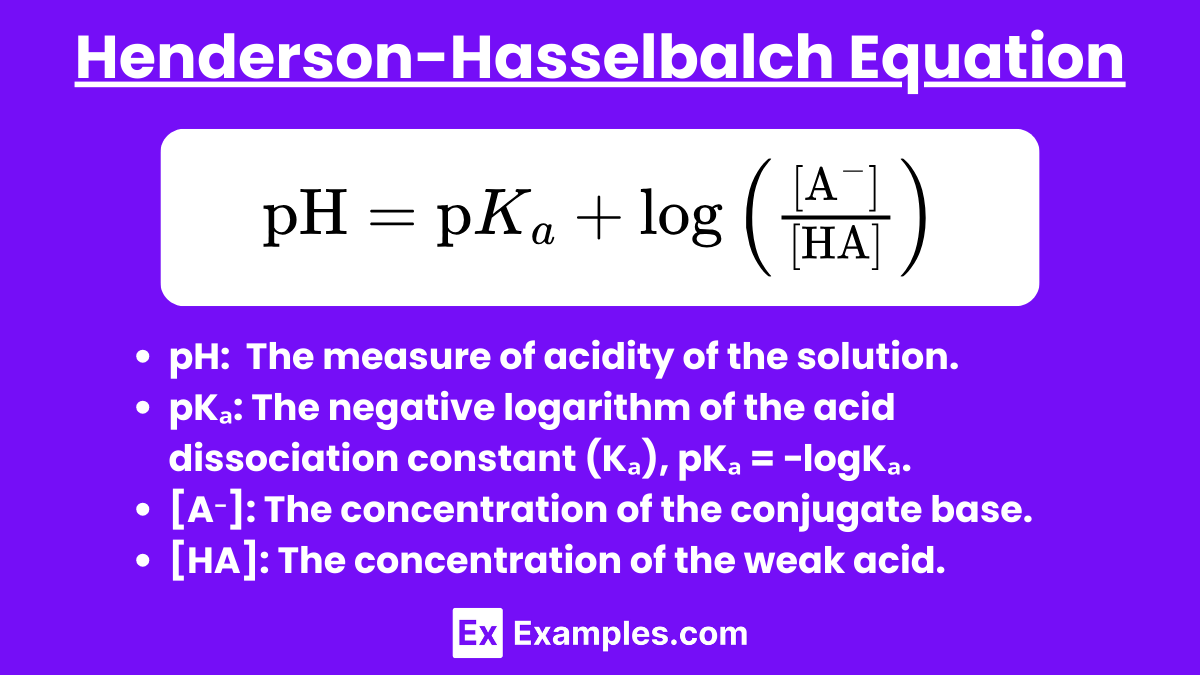
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of a buffer solution. It relates the pH of the solution to the concentration of the weak acid and its conjugate base (or weak base and its conjugate acid) and the acid dissociation constant (Kₐ).
Equation
For a buffer solution containing a weak acid (HA) and its conjugate base (A⁻), the equation is:
pH: The measure of acidity of the solution.
pKₐ: The negative logarithm of the acid dissociation constant (Kₐ), pKₐ = −logKₐ.
[A⁻]: The concentration of the conjugate base.
[HA]: The concentration of the weak acid.
Buffer Capacity
Buffer capacity is the ability of a buffer solution to resist changes in pH upon the addition of small amounts of an acid or a base. It measures how well the buffer can neutralize the added acid or base without a significant change in pH.
Factors Affecting Buffer Capacity
Concentration of Buffer Components:
Higher concentrations of the weak acid and its conjugate base (or weak base and its conjugate acid) result in greater buffer capacity.
A buffer with equal concentrations of the acid and conjugate base has optimal buffer capacity.
Ratio of Acid to Conjugate Base:
The buffer capacity is highest when the ratio of the acid to its conjugate base is close to 1:1.
A significant imbalance in this ratio reduces the buffer capacity.
Acid-Base Titrations
Acid-base titrations are analytical procedures used to determine the concentration of an unknown acid or base by reacting it with a solution of known concentration, called the titrant. The point at which the reaction is complete is known as the equivalence point, which can be detected using a pH indicator or a pH meter.
Titration Curve
A titration curve is a plot of the pH of the solution versus the volume of titrant added. The shape of the curve provides important information about the titration process and the nature of the acid or base being titrated.
Types of Acid-Base Titrations
1. Strong Acid-Strong Base Titration
Characteristics:
The pH starts low and rises sharply at the equivalence point.
The equivalence point is at pH 7.
2. Weak Acid-Strong Base Titration
Characteristics:
The pH starts higher than that of a strong acid and rises gradually.
The equivalence point is above pH 7.
3. Strong Acid-Weak Base Titration
Characteristics:
The pH starts low and increases gradually.
The equivalence point is below pH 7.
Indicators
Indicators are substances that change color at specific pH levels and are used to detect the equivalence point in a titration.
Common Indicators:
Phenolphthalein: Colorless in acidic solutions and pink in basic solutions. Suitable for strong acid-strong base titrations.
Methyl Orange: Red in acidic solutions and yellow in basic solutions. Suitable for strong acid-weak base titrations.