Learning Objectives
By studying Electrolysis and Faraday’s Law for the AP Chemistry exam, you should aim to understand the fundamental principles of electrolysis, including the roles of the electrolyte, anode, and cathode. You should be able to explain how electrical energy drives non-spontaneous redox reactions, and calculate the amount of substances produced using Faraday’s laws. This includes determining the mass of elements deposited at electrodes and the volume of gases evolved, based on the current and time applied. Additionally, you should become proficient in writing balanced half-reactions for oxidation and reduction processes, and accurately use Faraday’s constant in quantitative problems.
Introduction
Electrolysis is a chemical process that uses an electric current to drive a non-spontaneous chemical reaction, often resulting in the decomposition of compounds. Faraday’s Laws of Electrolysis describe the quantitative relationship between the amount of substance produced at each electrode and the quantity of electric charge passed through the electrolyte. These laws state that the amount of chemical change is directly proportional to the electric current and the duration it flows, and that the amounts of different substances produced by the same quantity of electricity are proportional to their equivalent weights.
What is Electrolysis?
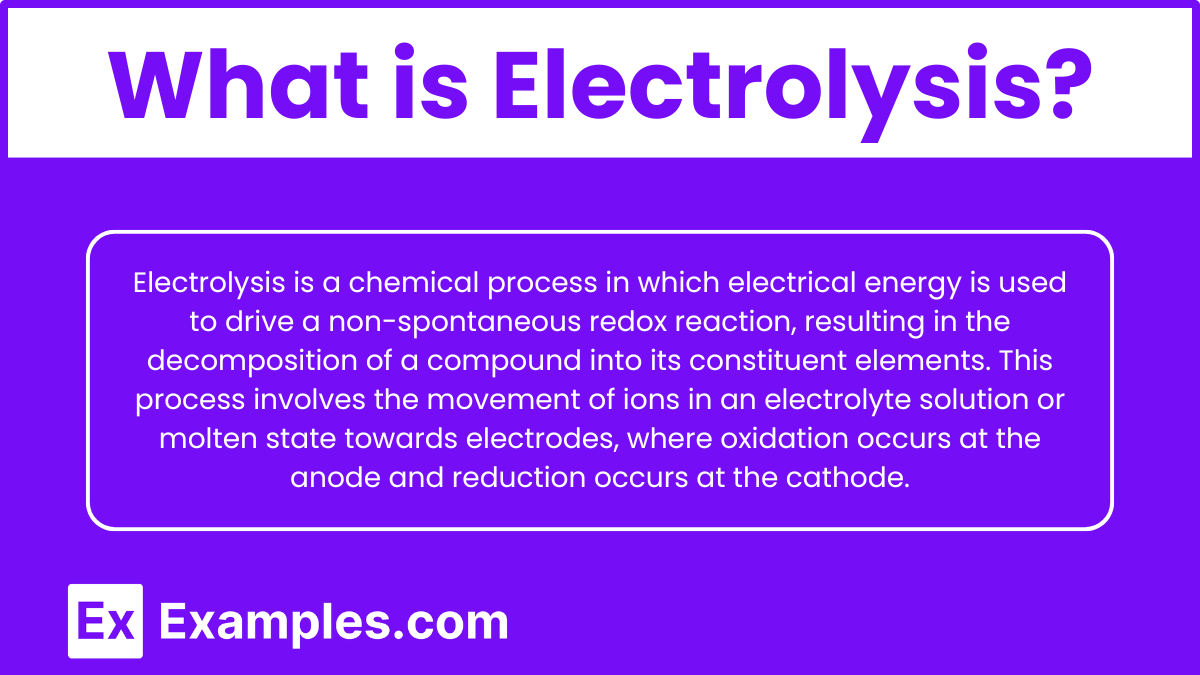
Electrolysis is a chemical process in which electrical energy is used to drive a non-spontaneous redox reaction, resulting in the decomposition of a compound into its constituent elements. This process involves the movement of ions in an electrolyte solution or molten state towards electrodes, where oxidation occurs at the anode and reduction occurs at the cathode.
Key Components of Electrolysis
- Electrolyte:
- A substance that conducts electricity when molten or dissolved in water, allowing the flow of ions.
- Common electrolytes include salts, acids and bases.
- Electrodes:
- Conductors that allow electric current to enter or leave the electrolyte.
- Anode: Positive electrode where oxidation occurs (loss of electrons).
- Cathode: Negative electrode where reduction occurs (gain of electrons).
- Typically made of inert materials like platinum or graphite, but can also be reactive depending on the specific electrolysis process.
- Power Source:
- Provides the necessary electrical energy to drive the non-spontaneous reaction.
- Common power sources include batteries or direct current (DC) power supplies.
- Electrolytic Cell:
- The entire setup where electrolysis takes place, including the container, electrolyte, electrodes, and power source.
- Designed to facilitate the efficient flow of electric current through the electrolyte and allow for the collection of products formed at the electrodes.
- Ion Movement:
- Cations (positively charged ions) move towards the cathode to gain electrons (reduction).
- Anions (negatively charged ions) move towards the anode to lose electrons (oxidation).
Common Electrolysis Reactions
1. Electrolysis of Water:
Reaction:![]()
Half-reactions:
- At the Cathode (Reduction):
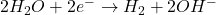
- At the Anode (Oxidation):
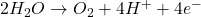
2. Electrolysis of Molten Sodium Chloride:
Reaction:![]()
Half-reactions:
- At the Cathode (Reduction):
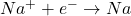
- At the Anode (Oxidation):
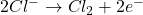
Faraday’s Laws of Electrolysis
Faraday’s First Law of Electrolysis
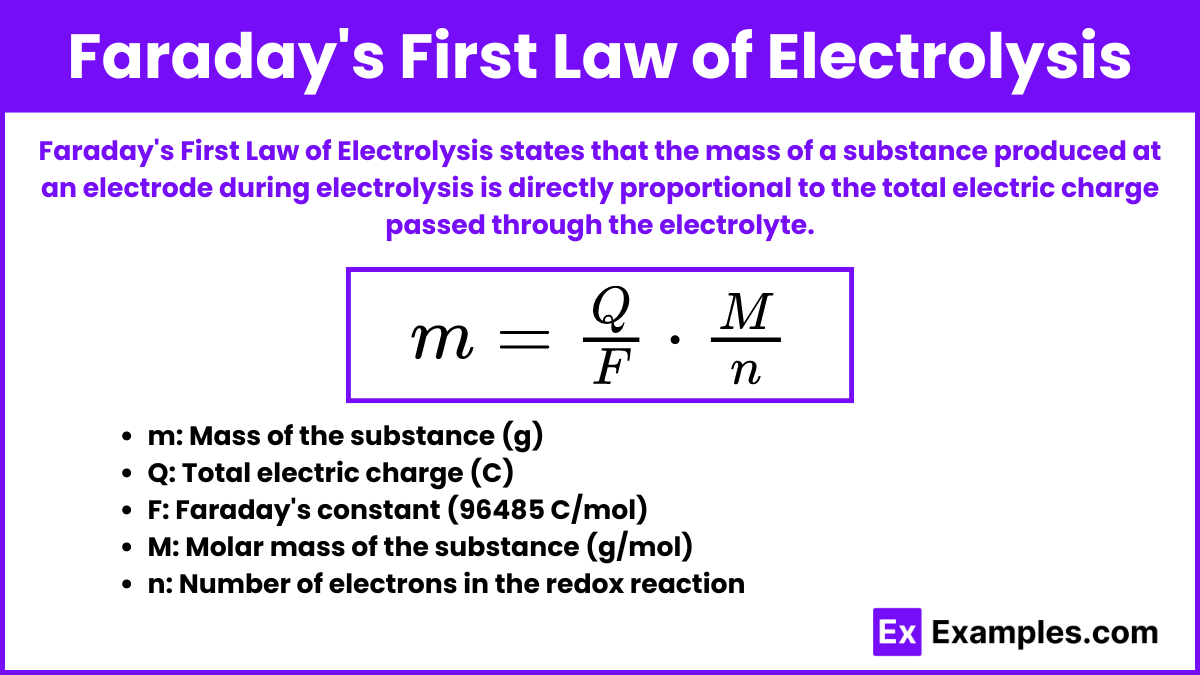
Faraday’s First Law of Electrolysis states that the mass of a substance produced at an electrode during electrolysis is directly proportional to the total electric charge passed through the electrolyte.
Mathematical Expression:
![]()
Where:
- m is the mass of the substance produced (in grams).
- Q is the total electric charge passed through the electrolyte (in coulombs).
- F is Faraday’s constant (96485, C/mol).
- M is the molar mass of the substance (in grams per mole).
- n is the number of electrons involved in the redox reaction.
Key Points to Understand:
- Electric Charge (Q):
The charge is calculated using the current (I) and the time (t) for which the current is applied.
Q= I x T
Where: - I is the current (in amperes).
- t is the time (in seconds).
- Faraday’s Constant (F):
Represents the charge of one mole of electrons, approximately ( 96485, C/mol ). - Molar Mass (M):
The mass of one mole of the substance being produced. - Number of Electrons (n):
The number of electrons required to produce one molecule or atom of the substance.
Example Problem:
Problem: Calculate the mass of silver deposited at the cathode when a current of 3.0 A is passed through a solution of silver nitrate (AgNO₃) for 2 hours.
Solution:
- Calculate the total charge (Q):
Q = I × t = 3.0A × (2 × 3600s) = 21600C - Determine the molar mass (M) of silver (Ag):
M = 107.87g/mol - Identify the number of electrons (n):
For Ag⁺ + e⁻ → Ag, n = 1 - Apply Faraday’s First Law:
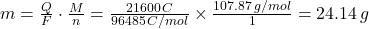
Therefore, 24.14 grams of silver will be deposited at the cathode.
Faraday’s Second Law of Electrolysis
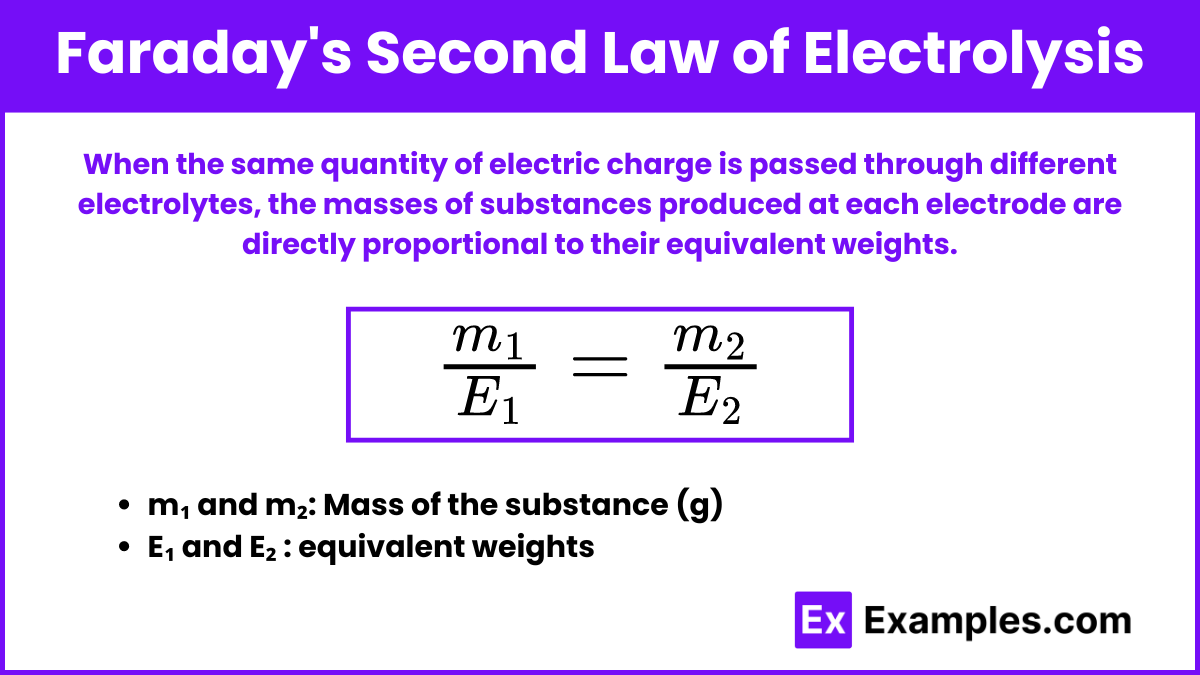
Faraday’s Second Law of Electrolysis states that when the same quantity of electric charge is passed through different electrolytes, the masses of substances produced at each electrode are directly proportional to their equivalent weights.
Key Points to Understand:
- Equivalent Weight (E):
Equivalent weight is calculated as the molar mass of the substance divided by the number of electrons involved in the redox reaction.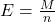
Where: - M is the molar mass of the substance (in grams per mole).
- n is the number of electrons involved in the reaction.
Mathematical Expression:
When the same charge ( Q ) is passed through different electrolytes, the masses m₁ and m₂ of substances deposited or liberated are proportional to their equivalent weights E₁ and E₂:![]()
Example Problem:
Problem: If a current is passed through solutions of copper sulfate (CuSO₄) and silver nitrate (AgNO₃) and 0.5 grams of copper is deposited, how much silver will be deposited in the same time?
- Determine the equivalent weight of copper (Cu):
- Molar mass of Cu: 63.55 g/mol
- Number of electrons (n) in the reaction Cu²⁺ + 2e⁻ → Cu: n=2
- Equivalent weight of Cu:
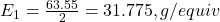
- Determine the equivalent weight of silver (Ag):
- Molar mass of Ag: 107.87 g/mol
- Number of electrons (n) in the reaction Ag⁺ + e⁻ → Ag : n = 1
- Equivalent weight of Ag:
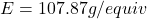
- Using Faraday’s Second Law:
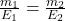 Where:
Where:
- m₁ = 0.5 g (mass of copper deposited)
- E₁ = 31.775 g/equiv (equivalent weight of copper)
- E₂ = 107.87 g/equiv (equivalent weight of silver)
- Solve for m₂(mass of silver deposited):
Therefore, 1.70 grams of silver will be deposited when 0.5 grams of copper is deposited under the same conditions.
Applications of Electrolysis
- Electroplating: Coating objects with thin layers of metal (e.g., gold plating on jewelry).
- Extraction of Metals: Extracting reactive metals from ores (e.g., aluminum from bauxite).
- Electrorefining: Purifying metals (e.g., refining copper).
- Production of Chemicals: Producing industrial chemicals (e.g., chlorine and sodium hydroxide from brine).
- Water Splitting: Producing hydrogen and oxygen from water.
- Electrolytic Cleaning: Removing rust and impurities from metal objects.
- Electrosynthesis: Synthesizing complex compounds (e.g., organic compounds like adiponitrile).
- Anodization: Increasing the oxide layer on metals (e.g., anodizing aluminum).
- Medical Applications: Medical treatments and procedures (e.g., galvanic therapy, hair removal).
- Fuel Cells and Batteries: Operating fuel cells and rechargeable batteries (e.g., lithium-ion batteries).