Learning Objectives
In the topic of Elementary Reactions for the AP Chemistry exam, you should learn to define and identify elementary reactions, understand and explain their characteristics, and distinguish between unimolecular, bimolecular, and termolecular reactions based on molecularity. You should be able to formulate and interpret rate laws directly from the stoichiometry of elementary reactions, differentiate elementary reactions from non-elementary reactions, and use experimental data to validate the proposed mechanisms. Mastery of these concepts includes applying knowledge to solve problems and analyze reaction mechanisms accurately, ensuring a comprehensive understanding of chemical kinetics.
Free AP Chemistry Practice Test
Introductions
Elementary reactions are fundamental processes in chemical kinetics, involving a single step where reactants directly convert to products. These reactions occur without intermediates and typically involve a small number of molecules. Characterized by their simplicity, elementary reactions can be unimolecular, bimolecular, or termolecular, depending on the number of reacting species. Understanding these reactions is crucial as they form the basis for more complex reaction mechanisms and help in elucidating reaction rates, molecular dynamics, and energy changes within a system.
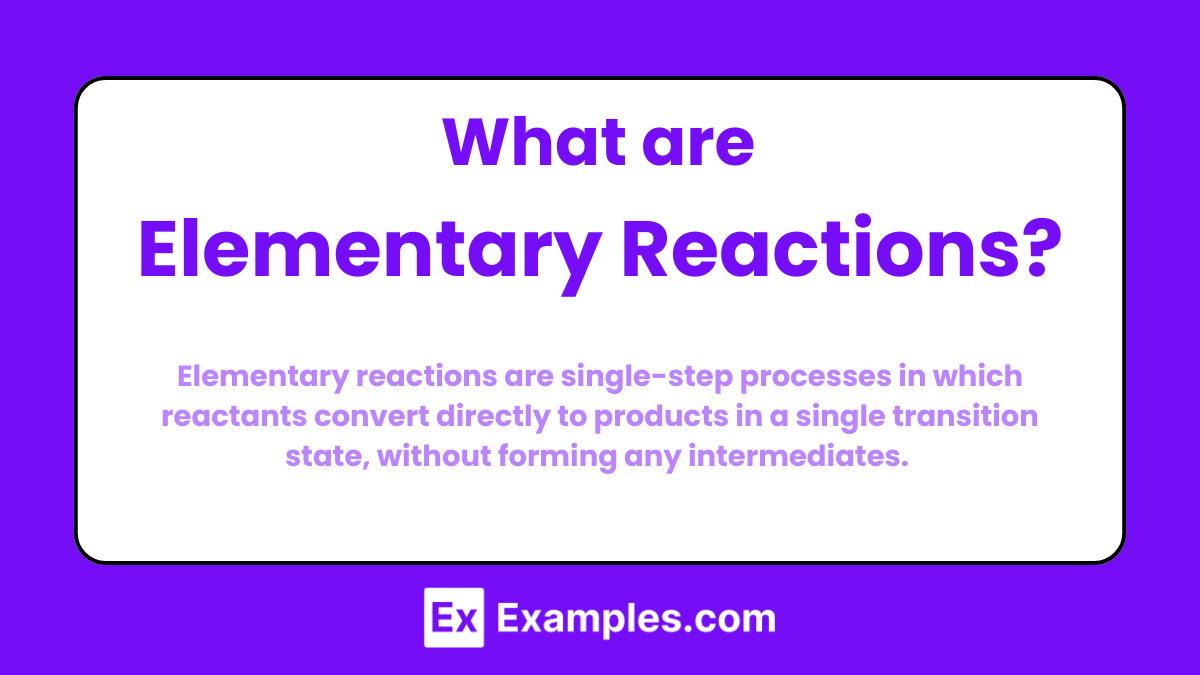
What are Elementary Reactions?
Elementary reactions are single-step processes in which reactants convert directly to products in a single transition state, without forming any intermediates. The rate law for an elementary reaction can be directly written from its balanced chemical equation, and the molecularity corresponds to the number of reactant molecules involved in the reaction.
Characteristics of Elementary Reactions
Single-Step Process: Occur in a single step without intermediates; reactants directly form products in one transition state.
Molecularity: Number of reactant molecules involved.
Unimolecular: Single reactant molecule (A → Products).
Bimolecular: Collision of two reactant molecules (A + B → Products).
Termolecular: Collision of three reactant molecules (rare, A + B + C → Products).
Rate Law from Stoichiometry: Can be written directly from the balanced chemical equation.
Unimolecular Reaction: Rate = k[A].
Bimolecular Reaction: Rate = k[A][B].
Transition State: Highest energy state during the conversion of reactants to products; represents the point of highest energy along the reaction path.
Activation Energy (Ea): Minimum energy required for reactants to reach the transition state and form products; higher activation energy means a slower reaction.
Reaction Coordinate Diagrams: Visualize energy changes during a reaction.
Components: Reactants (starting point), Transition State (peak energy), Products (end point).
No Intermediates: Do not involve intermediate species; proceed directly from reactants to products.
Collision Theory: Reactions occur when molecules collide with sufficient energy and proper orientation; explains activation energy and orientation factor in bimolecular and termolecular reactions.
Reaction Order Equals Molecularity: Order corresponds to molecularity.
Unimolecular: First-order reaction.
Bimolecular: Second-order reaction.
Reversibility: Many are reversible, with the reverse reaction also being elementary (A → B and B → A).
Steps to Analyze Elementary Reactions
Identify Reactants and Products: Determine the species involved.
Write Molecularity: Based on the number of reactants.
Formulate Rate Law: Use the molecularity to write the rate law.
Verify Experimental Data: Ensure the rate law matches experimental observations.
Molecularity of Elementary Reactions
Molecularity refers to the number of reactant molecules involved in an elementary reaction.
Types of Molecularity
Unimolecular Reactions
Involve a single reactant molecule.
Example: A → Products.
Bimolecular Reactions
Involve the collision of two reactant molecules.
Example: A + B → Products.
Termolecular Reactions
Involve the collision of three reactant molecules (rare).
Example: A + B + C → Products.
Rate Laws for Elementary Reactions
Rate laws for elementary reactions describe how the rate of reaction depends on the concentration of reactants. For elementary reactions, the rate law can be directly derived from the balanced chemical equation.
Unimolecular Reactions
Rate Law: Rate = k[A]
Bimolecular Reactions
Rate Law: Rate = k[A][B]
Termolecular Reactions
Rate Law: Rate = k[A][B][C]
Determining Molecularity from Rate Law
Molecularity refers to the number of reactant molecules involved in an elementary reaction.
Steps to Determine Molecularity from Rate Law
Identify the Rate Law:
Express the rate law in the form Rate = k[A]ᵐ[B]ⁿ, where m and n are the reaction orders with respect to each reactant.
Sum the Exponents:
Add the exponents of the reactant concentrations in the rate law.
Total Molecularity = m + n.
Reaction Coordinate Diagram
A reaction coordinate diagram visualizes the energy changes during a chemical reaction, plotting the progress of the reaction against the potential energy of the system. The diagram typically includes:
Reactants: The starting materials, shown at the beginning of the diagram.
Transition State: The highest energy point, representing the peak of the energy barrier that must be overcome for the reaction to proceed.
Activation Energy (Ea): The energy difference between the reactants and the transition state.
Products: The final substances, shown at a lower energy level than the reactants if the reaction is exothermic, or at a higher energy level if the reaction is endothermic.
Importance in Reaction Mechanisms
Elementary reactions are crucial for understanding the detailed pathway of how reactants convert to products. They help determine the rate law, identify intermediates, and predict reaction behavior. This knowledge aids in optimizing reaction conditions, designing synthetic routes, and developing catalysts to control and enhance chemical processes.
Distinguishing Elementary from Non-Elementary Reactions
Characteristic | Elementary Reactions | Non-Elementary Reactions |
|---|---|---|
Definition | Single-step processes with no intermediates | Consist of multiple steps with intermediates |
Molecularity | Determined by the stoichiometry of the reaction | Cannot be determined from overall stoichiometry |
Rate Law | Directly derived from the balanced equation | Not directly related to the balanced equation |
Rate-Determining Step | Only one step, which determines the rate | Involves multiple steps; the slowest step determines the rate |
Transition State | Only one transition state | Multiple transition states, one for each step |
Reaction Coordinate Diagram | Single energy peak (transition state) | Multiple energy peaks and valleys for intermediates and transition states |
Presence of Intermediates | None | Intermediates are formed and consumed |
Practice Problems
Problem 1: Identify Molecularity
Given the following reaction, determine its molecularity:
Solution:
This reaction involves a single molecule of N₂O₅.
Molecularity: Unimolecular.
Problem 2: Write the Rate Law
For the elementary reaction:
2NO₂ → 2NO + O₂
Write the rate law.
Solution:
The reaction involves two molecules of NO₂.
Rate Law: Rate = k[NO₂]².
Problem 3: Determine Molecularity from Rate Law
Given the rate law:
Rate = k[H₂][I₂]
Determine the molecularity of the reaction.
Solution:
The rate law indicates that the reaction involves one molecule of H₂ and one molecule of I₂.
Molecularity: Bimolecular.