Learning Objectives
By studying endothermic and exothermic processes for the AP Chemistry exam, you should aim to understand the definitions and characteristics of each process, including energy absorption and release, and the signs of enthalpy change (ΔH). You should be able to identify examples of each type of reaction, such as combustion for exothermic and photosynthesis for endothermic processes. Additionally, you should learn to interpret energy diagrams, use calorimetry to measure heat changes, and apply Hess’s Law to calculate the enthalpy changes of complex reactions. Finally, mastering the ability to write and balance thermochemical equations and recognizing the experimental indicators of heat transfer will solidify your understanding of these fundamental thermodynamic concepts.
Introduction
Endothermic and exothermic processes are fundamental concepts in chemistry that describe how energy is transferred during chemical reactions. In an endothermic process, the system absorbs energy from its surroundings, typically in the form of heat, resulting in a temperature decrease in the environment. Conversely, an exothermic process releases energy into the surroundings, often increasing the temperature of the environment. These processes are crucial in understanding reaction energetics, influencing everything from everyday phenomena like ice melting and combustion to industrial applications and biological functions.
Endothermic Processes
What are Endothermic Processes?
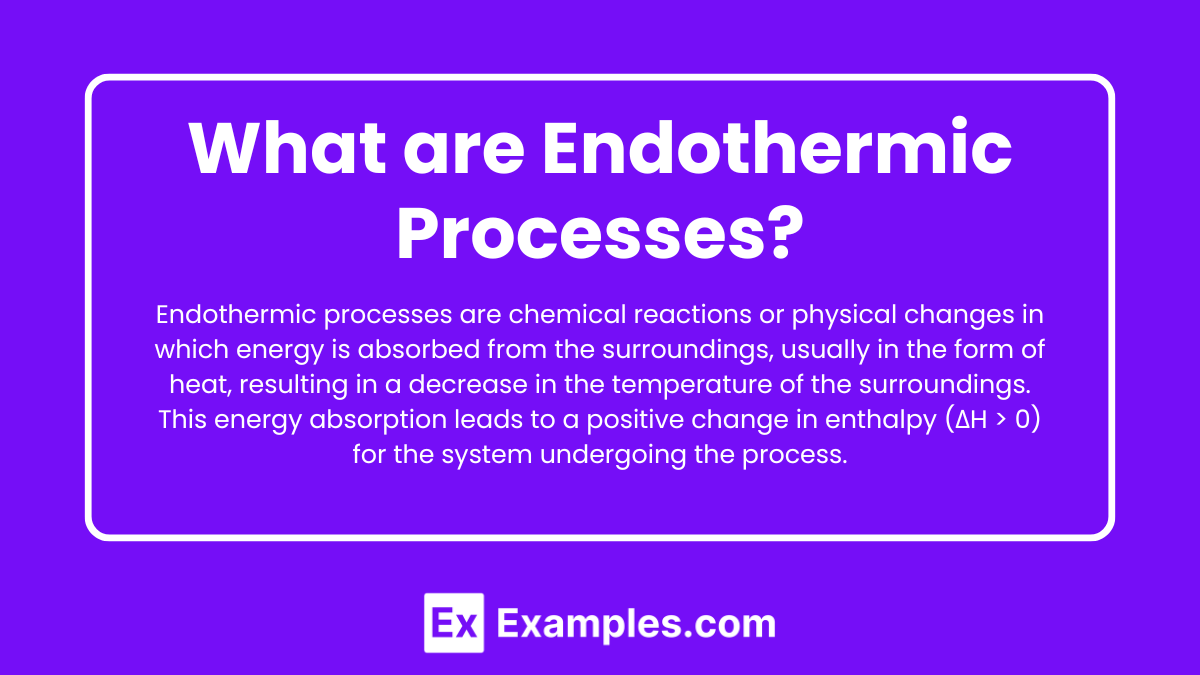
Endothermic processes are chemical reactions or physical changes in which energy is absorbed from the surroundings, usually in the form of heat, resulting in a decrease in the temperature of the surroundings. This energy absorption leads to a positive change in enthalpy (ΔH > 0) for the system undergoing the process.
Key Characteristics of Endothermic Processes
- Energy Absorption: Energy is absorbed from the surroundings.
- Positive ΔH: The enthalpy change (ΔH) is positive.
- Temperature Decrease: The temperature of the surroundings decreases.
- Phase Changes: Includes processes such as melting, vaporization, and sublimation.
- Non-spontaneous at Room Temperature: Often require an input of energy to occur.
Endothermic Reaction Graph
| Component | Description |
|---|---|
| Reactants | Lower energy level compared to products. |
| Products | Higher energy level; energy is absorbed from surroundings. |
| Energy Change | Represented by an upward arrow (energy absorbed). |
Examples of Endothermic Reaction
Photosynthesis:
- Reaction:

- Description: Plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen.
Melting Ice:
- Reaction:

- Description: Ice absorbs heat from the surroundings to melt into liquid water.
Exothermic Processes
What are Exothermic Processes?
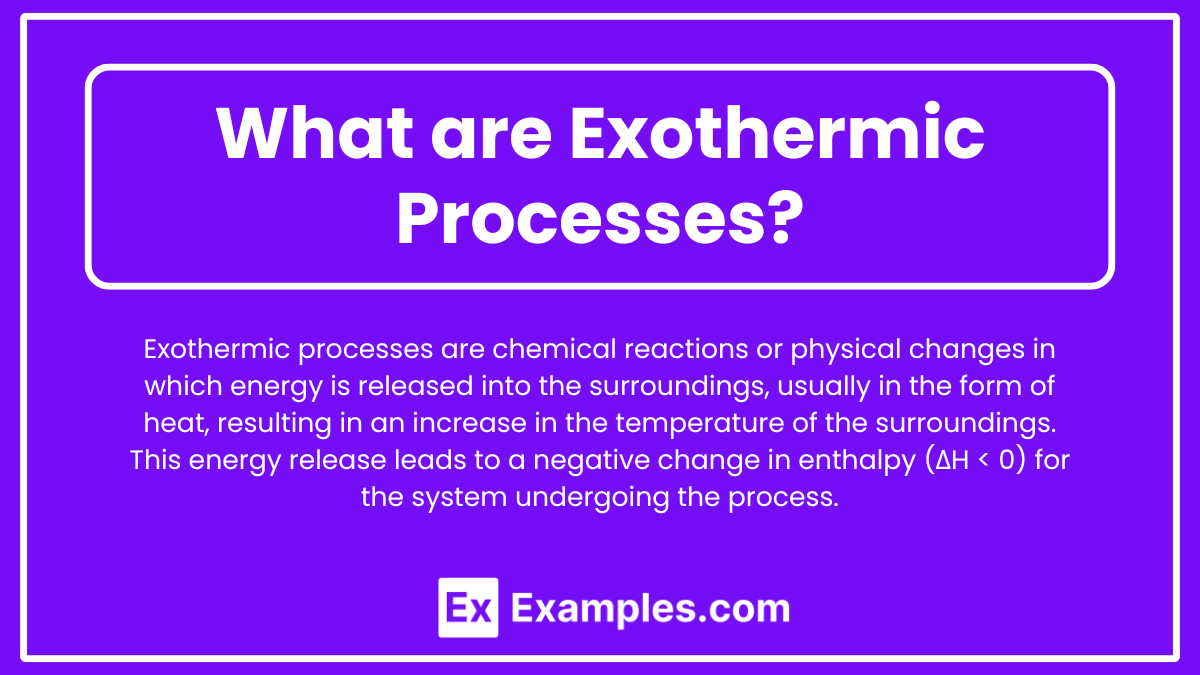
Exothermic processes are chemical reactions or physical changes in which energy is released into the surroundings, usually in the form of heat, resulting in an increase in the temperature of the surroundings. This energy release leads to a negative change in enthalpy (ΔH < 0) for the system undergoing the process.
Key Characteristics of Exothermic Processes
- Energy Release: Energy is released into the surroundings.
- Negative ΔH: The enthalpy change (ΔH) is negative.
- Temperature Increase: The temperature of the surroundings increases.
- Phase Changes: Includes processes such as freezing, condensation, and deposition.
- Spontaneous at Room Temperature: Often occur spontaneously under standard conditions.
Exothermic Reaction Graph
| Component | Description |
|---|---|
| Reactants | Higher energy level compared to products. |
| Products | Lower energy level; energy is released to surroundings. |
| Energy Change | Represented by a downward arrow (energy released). |
Examples of Exothermic Reaction
Combustion of Methane:
- Reaction:
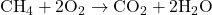
- Description: The combustion of methane releases a significant amount of energy in the form of heat and light.
Formation of Sodium Chloride:
- Reaction:

- Description: The reaction between sodium and chlorine to form sodium chloride releases energy, resulting in heat and light.
Identifying Endothermic and Exothermic Reactions
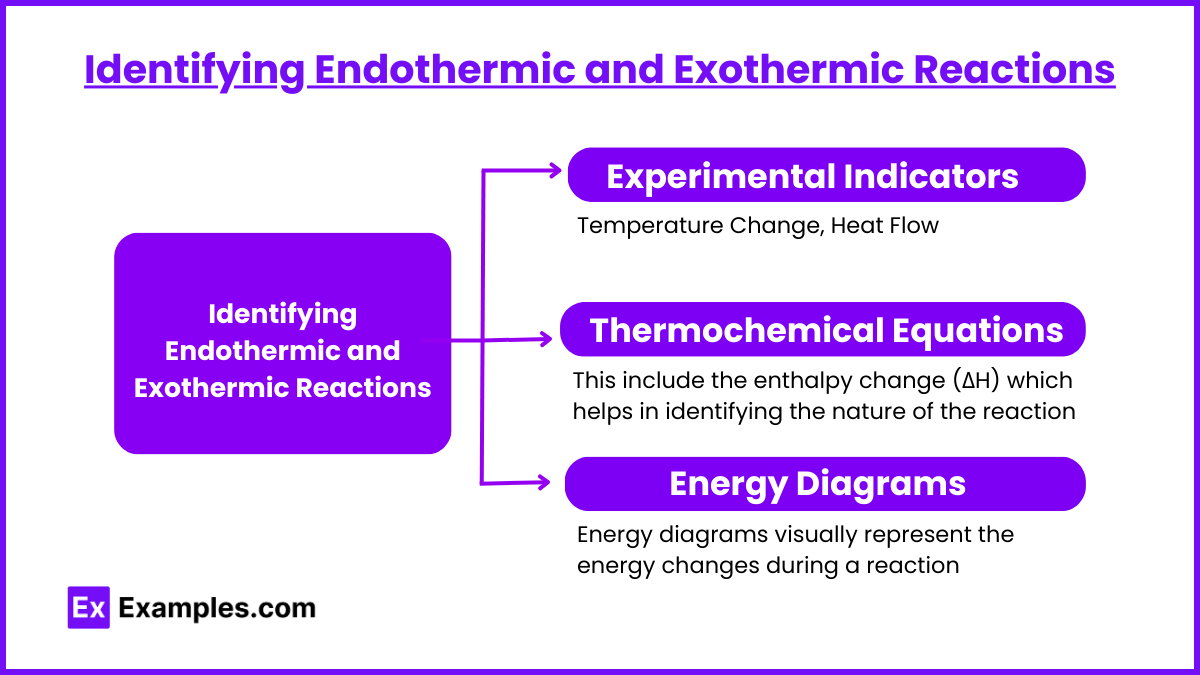
Experimental Indicators
- Temperature Change:
- Endothermic Reactions: Absorb heat from the surroundings, causing the temperature of the surroundings to decrease.
- Exothermic Reactions: Release heat to the surroundings, causing the temperature of the surroundings to increase.
- Heat Flow:
- Use a calorimeter to measure the heat flow.
- Endothermic Reactions: Heat flows from the surroundings into the system, causing the temperature of the calorimeter to decrease.
- Exothermic Reactions: Heat flows from the system into the surroundings, causing the temperature of the calorimeter to increase.
Thermochemical Equations
Thermochemical equations include the enthalpy change (ΔH) which helps in identifying the nature of the reaction:
- Exothermic Reactions: Have a negative ΔH value indicating that energy is released.
- Example:

- Example:
- Endothermic Reactions: Have a positive ΔH value indicating that energy is absorbed.
- Example:

- Example:
Energy Diagrams
Energy diagrams visually represent the energy changes during a reaction:
- Exothermic Reactions:
- Reactants start at a higher energy level.
- Products end at a lower energy level.
- Energy difference is shown as energy released to the surroundings.
- Endothermic Reactions:
- Reactants start at a lower energy level.
- Products end at a higher energy level.
- Energy difference is shown as energy absorbed from the surroundings.
Hess’s Law and Reaction Enthalpy
Hess’s Law states that the total enthalpy change of a reaction is the sum of the enthalpy changes for each step of the reaction, regardless of the path taken. This principle is based on the fact that enthalpy is a state function, meaning it depends only on the initial and final states of the system, not on the route taken to get from one to the other.
Applying Hess’s Law
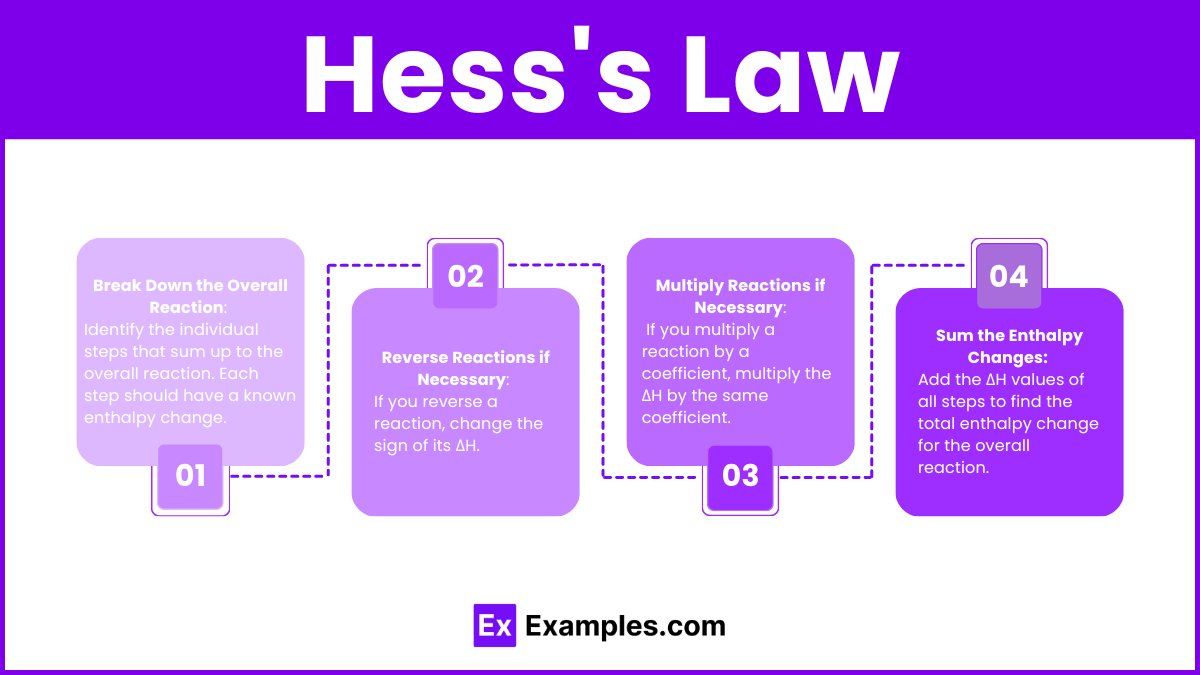
- Break Down the Overall Reaction: Identify the individual steps that sum up to the overall reaction. Each step should have a known enthalpy change.
- Reverse Reactions if Necessary: If you reverse a reaction, change the sign of its ΔH.
- Multiply Reactions if Necessary: If you multiply a reaction by a coefficient, multiply the ΔH by the same coefficient.
- Sum the Enthalpy Changes: Add the ΔH values of all steps to find the total enthalpy change for the overall reaction.
Example Problem
Given Reactions:
Overall Reaction:
![]()
Intermediate Steps (from methane formation):
Using Hess’s Law:
Reverse step 1 to decompose methane:
![]()
Add step 1 (reversed), step 2, and the given reactions:
![]()
![]()
Combine these with the given reactions to get:
![]()
- Reaction 1:

- Reaction 2:

Summing up:
ΔH = −74.8 + (−393.5) + (−483.6)
ΔH=−952 kJ/mol