Learning Objectives
For the AP Chemistry exam, you should focus on mastering the concept of enthalpy of formation. Understand how to define and calculate the standard enthalpy of formation (ΔHբ°) using enthalpies of reactants and products. Be proficient in applying Hess’s Law to determine the enthalpy changes in multi-step reactions. Recognize the difference between exothermic and endothermic reactions, and know how to interpret enthalpy changes in these contexts. Familiarize yourself with common enthalpies of formation for key compounds and practice problems involving bond enthalpies.
Introduction
The enthalpy of formation (ΔHբ°) is a fundamental concept in thermochemistry, representing the heat change that occurs when one mole of a compound is formed from its constituent elements in their standard states. It is a crucial parameter for understanding energy changes in chemical reactions and predicting reaction spontaneity. The standard enthalpy of formation is measured under standard conditions, typically at a pressure of 1 atmosphere and a temperature of 298 K (25°C). By utilizing enthalpy of formation values, chemists can calculate the enthalpy changes of various chemical reactions and design processes that maximize energy efficiency and safety.
What is Enthalpy of Formation?
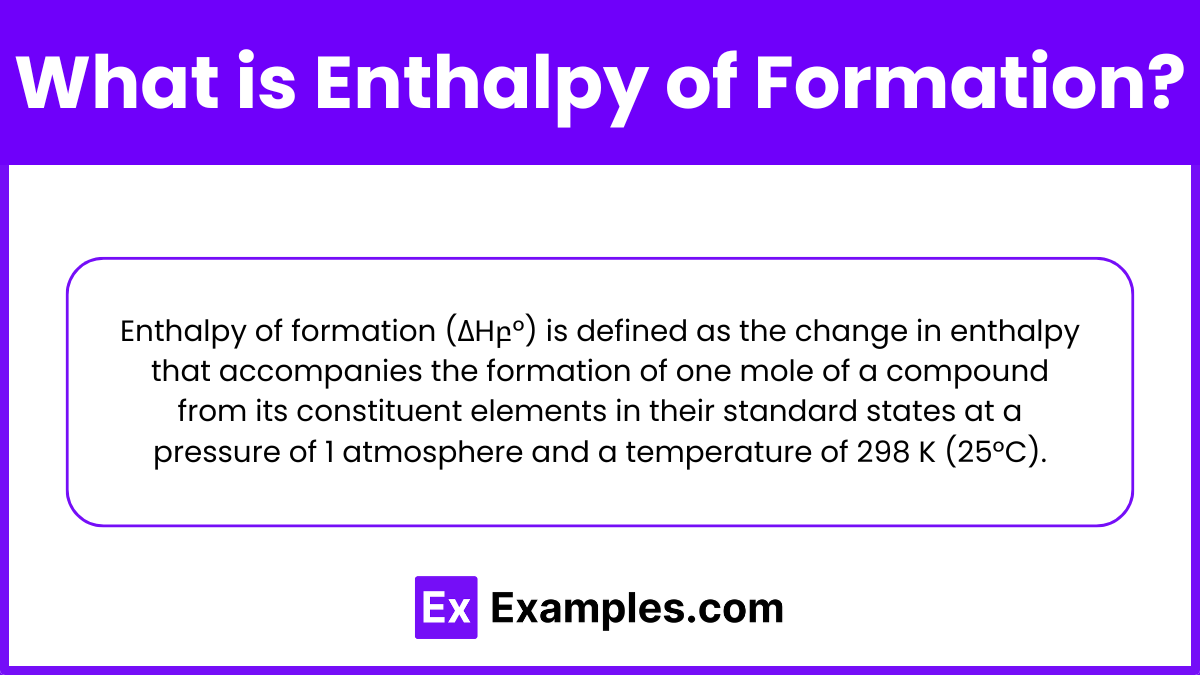
Enthalpy of formation (ΔHբ°) is defined as the change in enthalpy that accompanies the formation of one mole of a compound from its constituent elements in their standard states at a pressure of 1 atmosphere and a temperature of 298 K (25°C).
Standard States
Standard states refer to the most stable physical form of an element or compound under standard conditions of 1 atmosphere pressure and a temperature of 298 K (25°C). These states are used as reference points for thermodynamic calculations, including enthalpy of formation.
Examples of Standard States
- Gases: The standard state of a gas is the pure gas at 1 atm pressure.
- Example: Oxygen (O₂) as a gas.
- Liquids: The standard state of a liquid is the pure liquid at 1 atm pressure.
- Example: Water (H₂O) as a liquid.
- Solids: The standard state of a solid is the pure solid at 1 atm pressure.
- Example: Carbon (C) as graphite.
- Elements: For elements, the standard state is the form in which the element is most stable under standard conditions.
- Example: Nitrogen (N₂) as a gas, sulfur (S) as rhombic sulfur, and carbon (C) as graphite.
Formula for Enthalpy of Formation
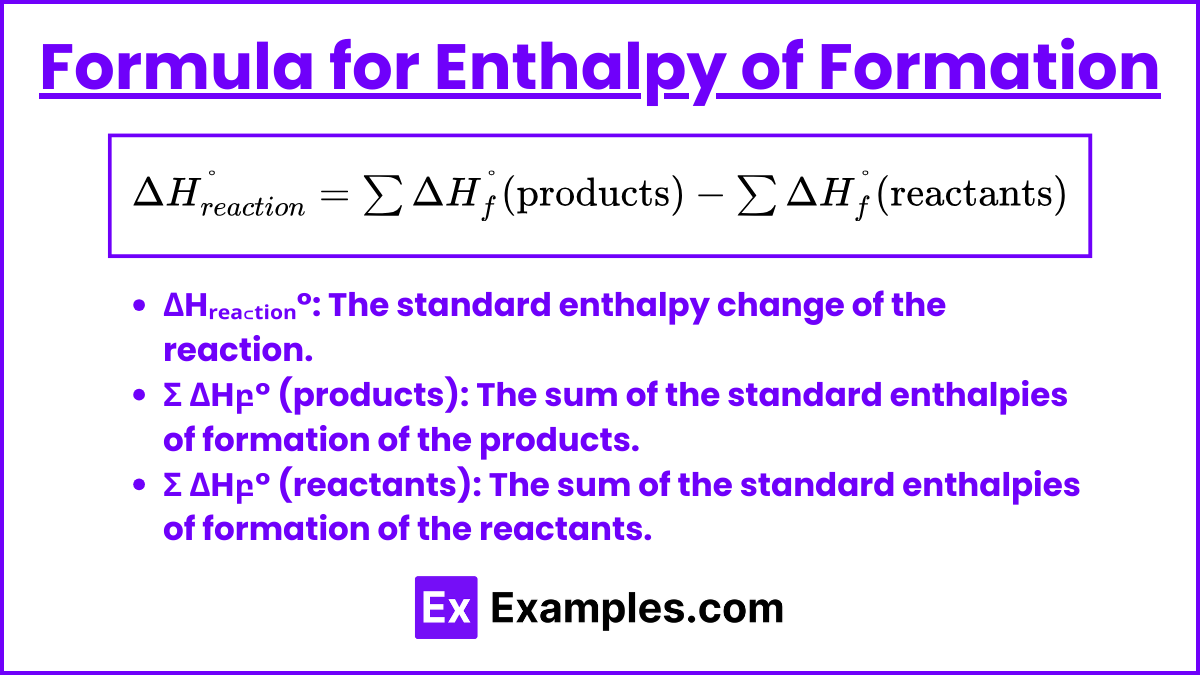
The enthalpy change for a reaction, which can include the formation of compounds from their elements, can be calculated using the standard enthalpies of formation (ΔH_f^°) of the reactants and products. The formula is:
Where,
- ΔHᵣₑₐ꜀ₜᵢₒₙ°: The standard enthalpy change of the reaction.
- Σ ΔHբ° (products): The sum of the standard enthalpies of formation of the products.
- Σ ΔHբ° (reactants): The sum of the standard enthalpies of formation of the reactants.
This formula calculates the overall enthalpy change of a reaction by subtracting the total enthalpy of the reactants from the total enthalpy of the products, both expressed in their standard states.
Calculating Enthalpy Change Using Enthalpy of Formation
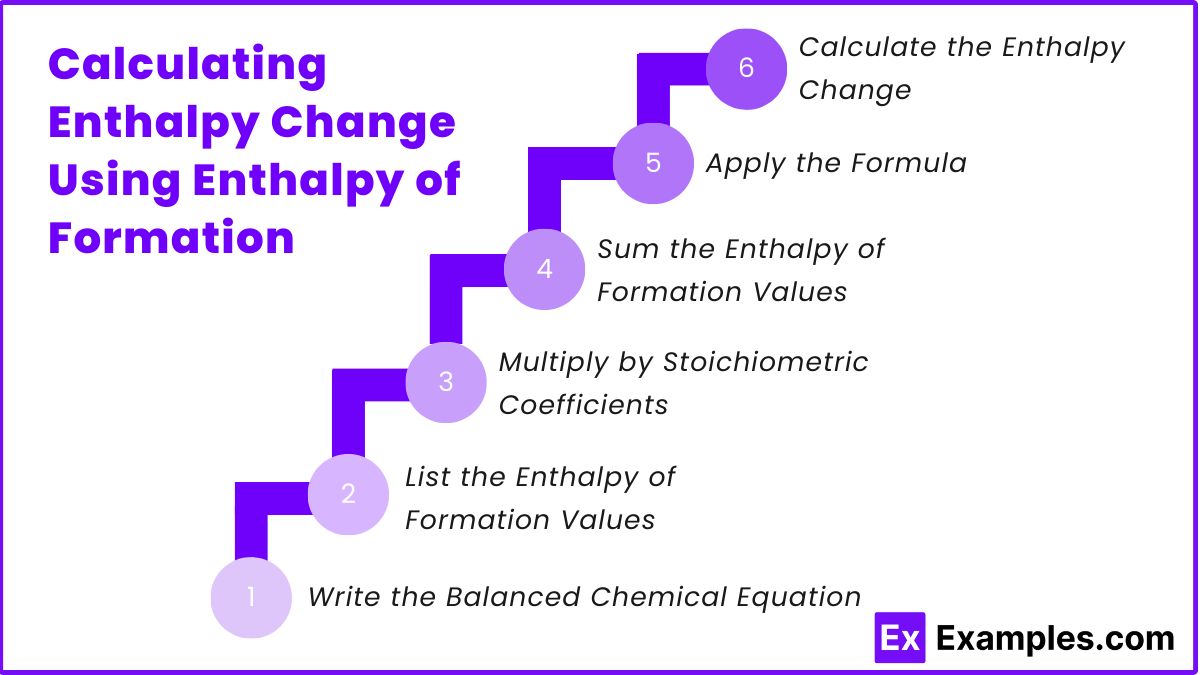
Write the Balanced Chemical Equation: Ensure the chemical equation is balanced to have the correct stoichiometric coefficients.
List the Enthalpy of Formation Values: Find the standard enthalpy of formation (ΔHբ°) values for all reactants and products involved in the reaction from a reliable source, such as a textbook or data table.
Multiply by Stoichiometric Coefficients: Multiply the ΔHբ° value of each reactant and product by its respective stoichiometric coefficient from the balanced chemical equation.
Sum the Enthalpy of Formation Values:
- Sum the enthalpy of formation values of all products.
- Sum the enthalpy of formation values of all reactants.
Apply the Formula: Use the formula to calculate the enthalpy change of the reaction:
Calculate the Enthalpy Change: Subtract the total enthalpy of the reactants from the total enthalpy of the products to find the standard enthalpy change of the reaction.
Standard Enthalpies of Formation
| Compound | ΔHբ° (kJ/mol) |
|---|---|
| H₂O(l) | -285.8 |
| CO₂(g) | -393.5 |
| CH₄(g) | -74.8 |
| NaCl(s) | -411.2 |
| NH₃(g) | -46.1 |
| C₂H₆(g) | -84.7 |
Example Calculation
Given Reaction: 2H₂(g) + O₂(g) → 2H₂O(l)
Enthalpy of Formation Values:
- ΔHբ°(H₂O(l)) = -285.8 kJ/mol
- ΔHբ°(H₂(g)) = 0 kJ/mol (standard state)
- ΔHբ°(O₂(g)) = 0 kJ/mol (standard state)
Steps:
- Balanced Equation: Already balanced.
- List Enthalpy Values:
- Products: 2 × ΔHբ°(H₂O(l)) = 2 × (-285.8 kJ/mol) = -571.6 kJ
- Reactants: 2 × ΔHբ°(H₂(g)) + 1 × ΔH_f^°(O₂(g)) = 2 × 0 + 1 × 0 = 0 kJ
- Sum Values:
- Products: -571.6 kJ
- Reactants: 0 kJ
- Apply Formula: ΔHᵣₑₐ꜀ₜᵢₒₙ° = −571.6 kJ − 0 kJ = −571.6 kJ
Thus, the enthalpy change for the formation of water from hydrogen and oxygen is -571.6 kJ.
Applications of Enthalpy of Formation
- Predicting Reaction Enthalpies: Calculate the enthalpy changes of chemical reactions using Hess’s Law.
- Designing Chemical Processes: Optimize industrial processes for energy efficiency and yield.
- Understanding Stability of Compounds: Assess the stability and reactivity of compounds.
- Environmental Chemistry: Evaluate the energy changes in pollutant formation and degradation.
- Combustion Reactions: Determine the heat released during combustion for energy production.
- Biochemical Reactions: Study metabolic pathways and energy transfer in living organisms.
- Materials Science: Design materials with specific thermal properties and energy storage capabilities.
- Thermodynamic Studies: Calculate other thermodynamic properties like Gibbs free energy and entropy.
- Chemical Education: Teach core concepts in thermodynamics and energy changes.
- Pharmaceutical Industry: Develop efficient synthetic routes for drug design and synthesis.