Learning Objectives
In studying Free Energy and Equilibrium for the AP Chemistry exam, you should aim to understand the definition and significance of Gibbs Free Energy (ΔG) and how it determines the spontaneity of chemical reactions. You should learn to calculate ΔG using the equation ΔG = ΔH - TΔS and understand the conditions under which reactions are spontaneous, non-spontaneous, or at equilibrium. Additionally, you should comprehend the relationship between ΔG° and the equilibrium constant (K) through the equation ΔG° = -RT ln K, and be able to interpret the values of ΔG° and K to predict reaction behavior. Lastly, you should grasp how Le Chatelier's Principle applies to changes in concentration, pressure, and temperature, and its impact on the equilibrium position of a reaction.
Free AP Chemistry Practice Test
Introduction
Free energy, specifically Gibbs free energy (G), helps predict if a chemical reaction will occur spontaneously. A reaction is spontaneous if it lowers the system's free energy. Equilibrium is the state where forward and reverse reactions happen at the same rate, with no net change in reactant and product concentrations. At equilibrium, the system's free energy is minimized, indicating a balanced state. These concepts are crucial for understanding and predicting chemical reaction behavior.
Gibbs Free Energy (G)
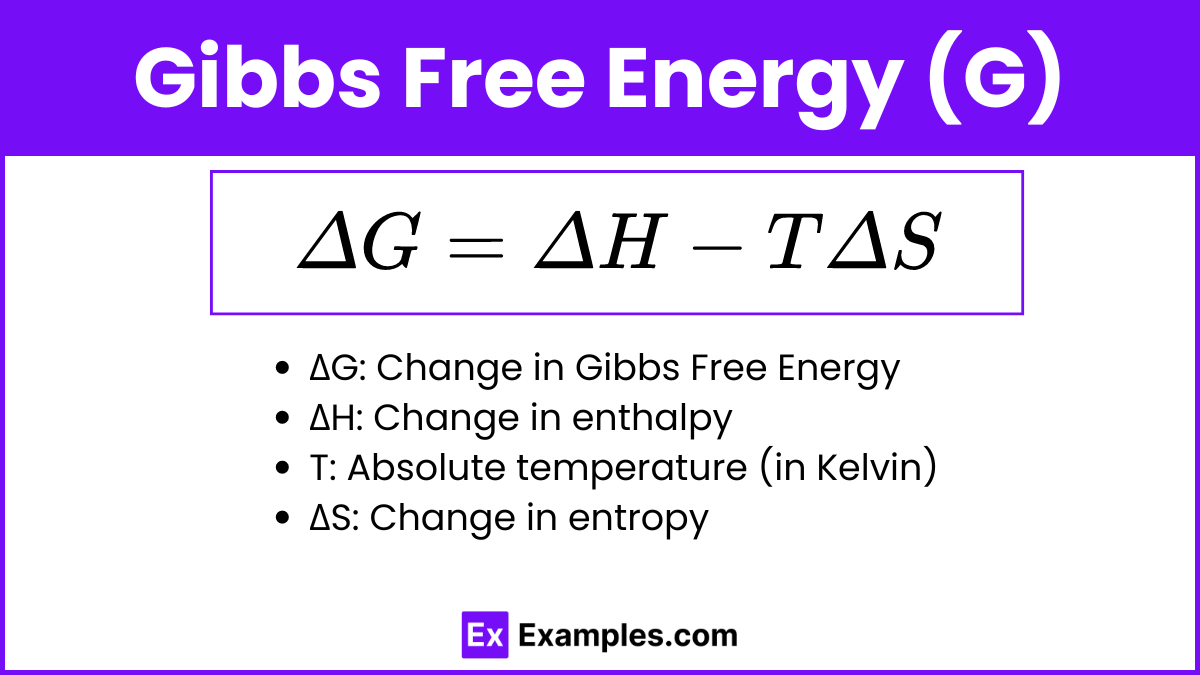
Gibbs Free Energy (G) is a thermodynamic potential that helps predict whether a chemical reaction will occur spontaneously. It combines enthalpy (H), which is the heat content, and entropy (S), which is the measure of disorder, at a constant temperature (T). The formula for calculating the change in Gibbs Free Energy is:
ΔG = ΔH − TΔS
ΔG: Change in Gibbs Free Energy
ΔH: Change in enthalpy
T: Absolute temperature (in Kelvin)
ΔS: Change in entropy
Significance of ΔG
ΔG < 0: The reaction is spontaneous (will proceed on its own).
ΔG > 0: The reaction is non-spontaneous (requires energy input to proceed).
ΔG = 0: The system is at equilibrium (no net change occurs).
Relationship Between Free Energy and Equilibrium
The relationship between free energy and equilibrium is essential for understanding how chemical reactions reach a state of balance.
Gibbs Free Energy and Equilibrium
At equilibrium, the change in Gibbs Free Energy (ΔG) is zero, indicating no net change in the concentration of reactants and products. The system is at its lowest free energy state, meaning that it is in a state of maximum stability.
Equilibrium Constant (K)
The equilibrium constant (K) expresses the ratio of the concentrations of products to reactants at equilibrium. It is a measure of the extent to which a reaction proceeds:
K = \frac{[products]}{[reactants]}
Standard Free Energy Change (ΔG°)
The standard free energy change (ΔG°) refers to the free energy change under standard conditions (1 M concentration, 1 atm pressure, and 25°C). The relationship between ΔG° and the equilibrium constant (K) is given by:
ΔG° = −RTlnK
R: Universal gas constant (8.314 J/mol·K)
T: Temperature in Kelvin
ln: Natural logarithm
K: Equilibrium constant
Interpretation of ΔG° and K
ΔG° < 0: K>1, meaning products are favored at equilibrium.
ΔG° > 0: K<1, meaning reactants are favored at equilibrium.
ΔG° = 0: K=1, meaning neither reactants nor products are favored; the system is perfectly balanced.
Calculating Free Energy Under Non-Standard Conditions
For reactions under non-standard conditions, the free energy change (ΔG) can be calculated using the reaction quotient (Q), which is analogous to K but for non-equilibrium conditions:
ΔG = ΔG° + RTlnQ
Q: Reaction quotient, which is calculated similarly to K but at any point in time.
Calculating Free Energy Changes
Calculating the change in Gibbs Free Energy (ΔG) is crucial for predicting whether a reaction will occur spontaneously, determining the equilibrium position, and understanding the effects of different conditions on a reaction.
Standard Free Energy Change (ΔG°)
The standard free energy change (ΔG°) is calculated under standard conditions (1 M concentration, 1 atm pressure, and 25°C):
ΔG° = ΔH − TΔS
ΔH: Change in enthalpy (standard heat of reaction)
T: Temperature in Kelvin (K)
ΔS: Change in entropy (standard entropy change)
Free Energy Change Under Non-Standard Conditions
To calculate the free energy change (ΔG) under non-standard conditions, we use the following equation that incorporates the reaction quotient (Q):
ΔG = ΔG° + RTlnQ
R: Universal gas constant (8.314 J/mol·K)
T: Temperature in Kelvin (K)
Q: Reaction quotient, calculated as the ratio of the concentrations of products to reactants at any point in time, similar to the equilibrium constant (K) but not necessarily at equilibrium.
Steps to Calculate ΔG
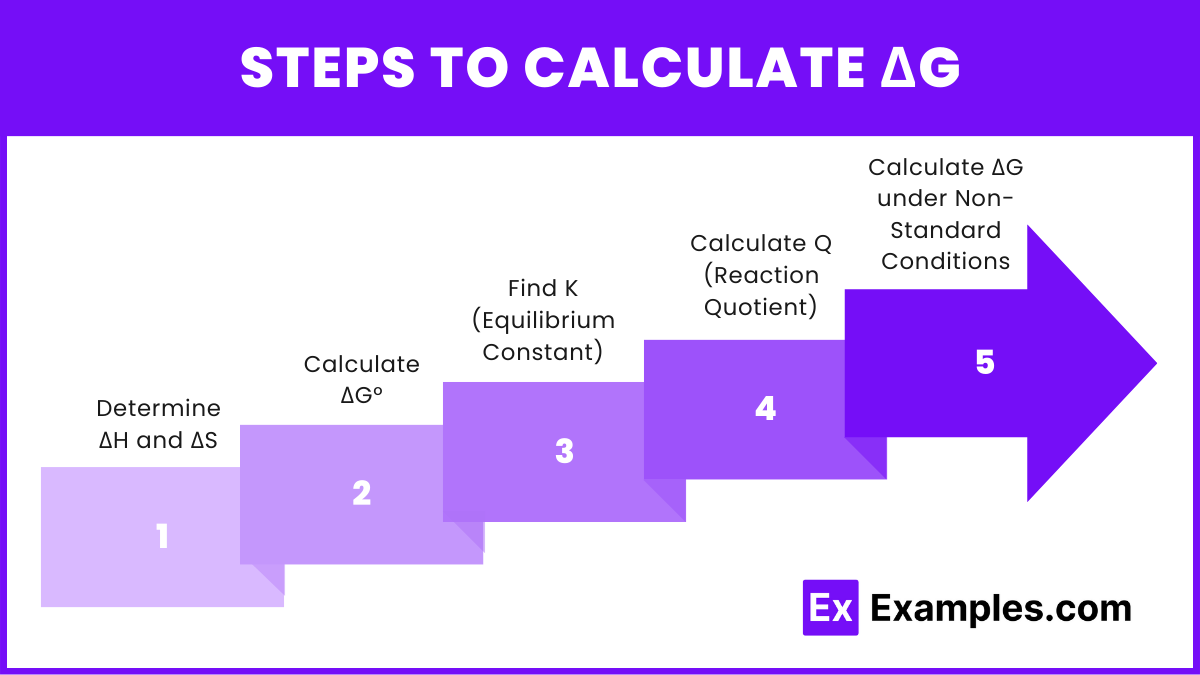
Determine ΔH and ΔS:
Use tabulated standard enthalpy and entropy values from reference sources or experimental data.
Calculate ΔG°:
Apply the formula ΔG° = ΔH − TΔS.
Find K (Equilibrium Constant):
If not given, you can derive K from ΔG° = −RTlnK.
Rearrange to K = e^{-\Delta G° / RT}.
Calculate Q (Reaction Quotient):
Determine the ratio of the concentrations of products to reactants at the specific point in time using the formula Q = \frac{[products]}{[reactants]}.
Calculate ΔG under Non-Standard Conditions:
Use the formula ΔG = ΔG° + RTlnQ.
Le Chatelier's Principle
Le Chatelier's Principle is a fundamental concept in chemistry that describes how a system at equilibrium responds to external changes. It states that if a dynamic equilibrium is disturbed by changing the conditions, the system shifts its position to counteract the disturbance and restore a new equilibrium.
Factors Affecting Equilibrium
1. Concentration Changes
Adding Reactants: If the concentration of reactants is increased, the system shifts to the right, favoring the formation of products to reduce the disturbance.
Removing Reactants: If the concentration of reactants is decreased, the system shifts to the left, favoring the formation of reactants.
Adding Products: If the concentration of products is increased, the system shifts to the left, favoring the formation of reactants.
Removing Products: If the concentration of products is decreased, the system shifts to the right, favoring the formation of products.
2. Pressure Changes
Increasing Pressure: If pressure is increased (by decreasing volume), the system shifts toward the side with fewer gas molecules to reduce pressure.
Decreasing Pressure: If pressure is decreased (by increasing volume), the system shifts toward the side with more gas molecules to increase pressure.
3. Temperature Changes
Exothermic Reactions (ΔH < 0): Increasing temperature shifts equilibrium to the left (favoring reactants), while decreasing temperature shifts equilibrium to the right (favoring products).
Endothermic Reactions (ΔH > 0): Increasing temperature shifts equilibrium to the right (favoring products), while decreasing temperature shifts equilibrium to the left (favoring reactants).