Learning Objectives
In studying Gibbs Free Energy and Thermodynamic Favorability for the AP Chemistry exam, you should aim to understand the definition and calculation of Gibbs free energy (G), the significance of the Gibbs free energy change (ΔG) in determining the spontaneity of reactions, and the relationships between enthalpy (ΔH), entropy (ΔS), and temperature in affecting ΔG. You should also be able to predict the thermodynamic favorability of reactions, interpret the meaning of positive, negative, and zero ΔG values, and understand the connection between ΔG and equilibrium constants (K). Mastery of these concepts involves performing calculations, analyzing reaction conditions, and applying these principles to various chemical scenarios.
Introduction
Gibbs Free Energy (G) is a key concept in chemistry that predicts whether a chemical reaction will occur spontaneously. It combines the system’s enthalpy (heat content) and entropy (degree of disorder). A negative Gibbs Free Energy (ΔG) means the reaction is thermodynamically favorable and will proceed on its own, while a positive ΔG indicates the reaction is not favorable. This concept is essential for understanding the feasibility of chemical reactions.
Gibbs Free Energy
What is Gibbs Free Energy?

Gibbs free energy (G) is a thermodynamic potential that measures the maximum amount of work that can be performed by a system at constant temperature and pressure. It is defined by the equation:
G = H − TS
where:
- G is the Gibbs free energy.
- H is the enthalpy.
- T is the absolute temperature in Kelvin.
- S is the entropy.
This quantity helps predict the direction of chemical reactions and the spontaneity of processes.
Gibbs Free Energy Change (ΔG)
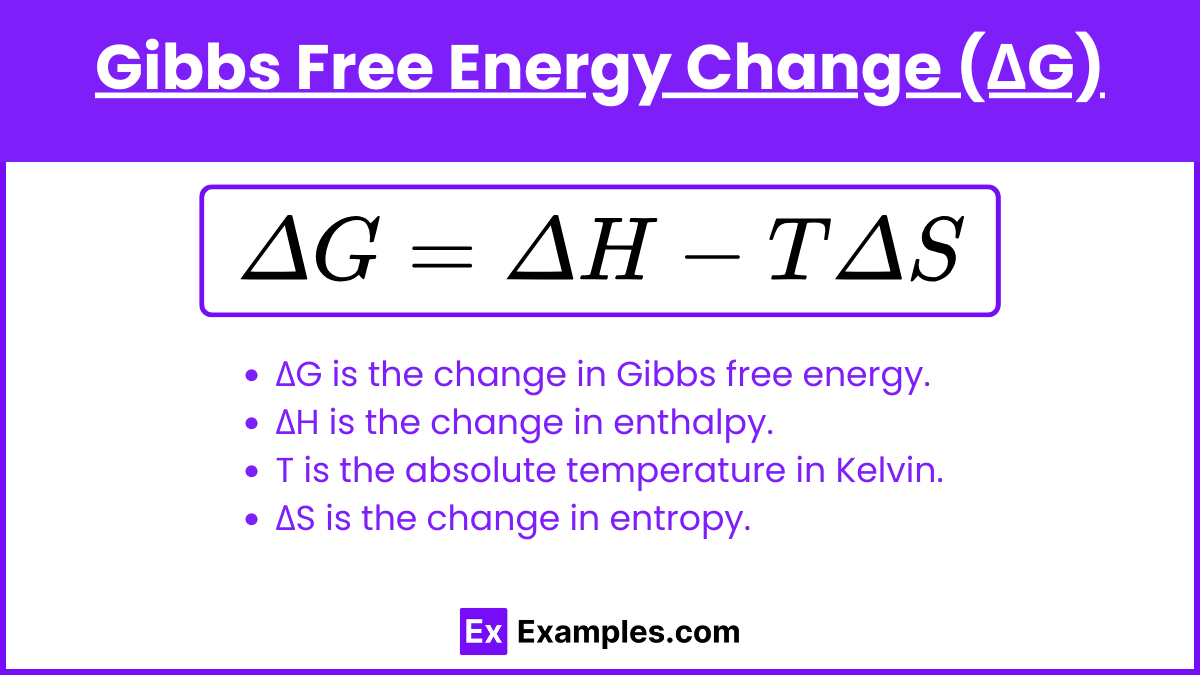
Gibbs free energy change (ΔG) is the difference in Gibbs free energy between the products and reactants of a chemical reaction. It indicates whether a reaction will occur spontaneously under constant temperature and pressure. The equation for Gibbs free energy change is:
ΔG = ΔH − TΔS
where:
- ΔG is the change in Gibbs free energy.
- ΔH is the change in enthalpy.
- T is the absolute temperature in Kelvin.
- ΔS is the change in entropy.
Interpretation of ΔG
- ΔG < 0: The reaction is spontaneous and thermodynamically favorable.
- ΔG = 0: The system is at equilibrium.
- ΔG > 0: The reaction is non-spontaneous and thermodynamically unfavorable.
Key Points
- Spontaneity: A negative ΔG implies a spontaneous process, meaning it can proceed without external energy input.
- Equilibrium: When ΔG = 0, the system has no net change and is at equilibrium.
- Non-Spontaneity: A positive ΔG indicates a non-spontaneous process, requiring energy input to proceed.
Calculating Gibbs Free Energy
Standard Gibbs Free Energy Change (ΔG∘)
The standard Gibbs free energy change (ΔG∘) is calculated under standard conditions (298 K, 1 atm, 1 M concentration). It can be determined using the standard enthalpy (ΔH∘) and standard entropy (ΔS∘) values with the equation:
ΔG∘ = ΔH∘ − TΔS∘
Non-Standard Conditions
For reactions not occurring under standard conditions, the Gibbs free energy change (ΔG) can be calculated using the reaction quotient (Q):
ΔG = ΔG∘ + RTlnQ
where:
- R is the gas constant (8.314 J/mol·K).
- T is the absolute temperature in Kelvin.
- Q is the reaction quotient.
Gibbs Free Energy and Equilibrium
At equilibrium, ΔG=0, and the relationship between the standard Gibbs free energy change and the equilibrium constant (K) is:
ΔG∘ = −RTlnK
Thermodynamic Favorability
What is Thermodynamic Favorability?
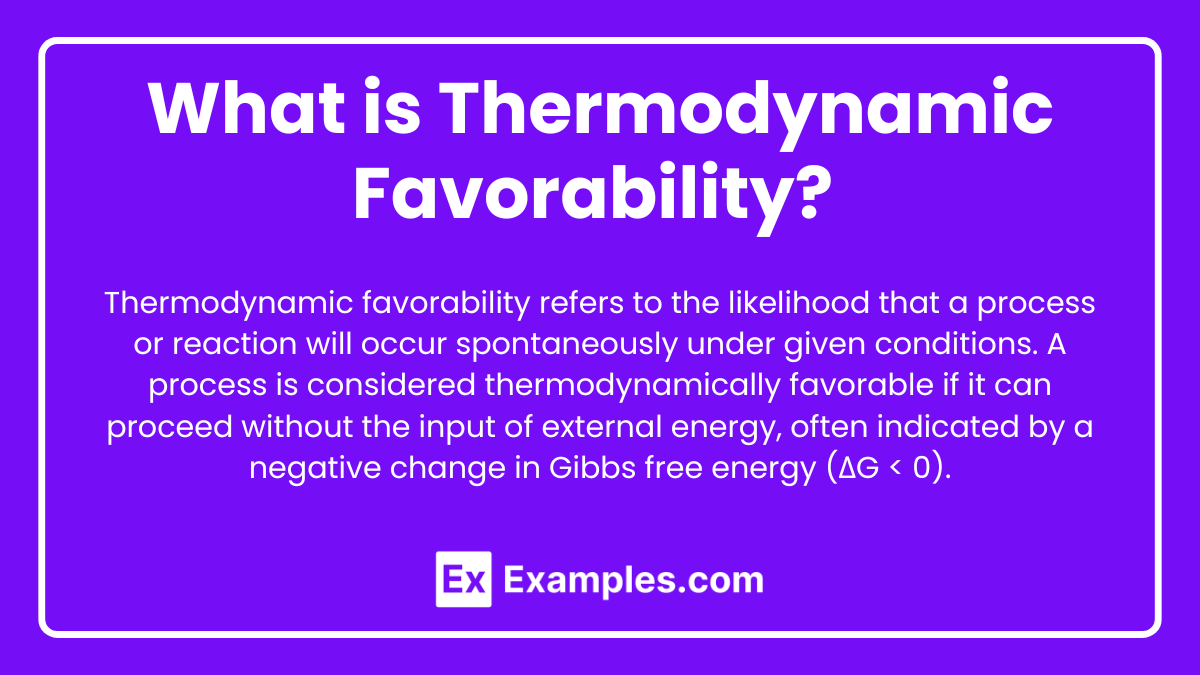
Thermodynamic favorability refers to the likelihood that a process or reaction will occur spontaneously under given conditions. A process is considered thermodynamically favorable if it can proceed without the input of external energy, often indicated by a negative change in Gibbs free energy (ΔG < 0).
Factors Affecting Thermodynamic Favorability
Thermodynamic favorability of a process is determined by the interplay of enthalpy (ΔH), entropy (ΔS), and temperature (T). The key factors are:
1. Enthalpy (ΔH)
- Exothermic Reactions (ΔH<0): These reactions release heat to the surroundings and are generally more favorable.
- Endothermic Reactions (ΔH>0): These reactions absorb heat from the surroundings and are generally less favorable.
2. Entropy (ΔS\Delta SΔS)
- Increase in Entropy (ΔS>0): Reactions that increase the disorder of the system tend to be more favorable.
- Decrease in Entropy (ΔS<0): Reactions that decrease the disorder of the system tend to be less favorable.
3. Temperature (T)
- Low Temperature: At lower temperatures, the enthalpy term (ΔH) has a greater impact on Gibbs free energy (ΔG).
- High Temperature: At higher temperatures, the entropy term (TΔS) becomes more significant.
Combined Effect on Gibbs Free Energy (ΔG)
The combined effect of these factors is summarized in the Gibbs free energy equation:
ΔG=ΔH−TΔS.
Scenarios
- ΔH<0 and ΔS>0: Reaction is always spontaneous (ΔG<0).
- ΔH<0 and ΔS<0: Reaction is spontaneous at low temperatures.
- ΔH>0 and ΔS>0: Reaction is spontaneous at high temperatures.
- ΔH>0 and ΔS<0: Reaction is never spontaneous (ΔG>0).
Temperature Dependence
The temperature dependence of Gibbs free energy (ΔG) plays a critical role in determining the spontaneity of a reaction. The effect of temperature on ΔG is dictated by the equation:
ΔG = ΔH − TΔS
where:
- ΔG is the Gibbs free energy change.
- ΔH is the enthalpy change.
- T is the absolute temperature in Kelvin.
- ΔS is the entropy change.
Key Scenarios
- Exothermic Reactions (ΔH<0) and Positive Entropy Change (ΔS>0):
- Always Spontaneous: These reactions are spontaneous at all temperatures because both terms (ΔH and −TΔS) contribute to making ΔG negative.
- Exothermic Reactions (ΔH<0) and Negative Entropy Change (ΔS<0):
- Spontaneous at Low Temperatures: These reactions are spontaneous at low temperatures where the negative ΔH term dominates. At higher temperatures, the −TΔS term becomes positive and can make ΔG positive, making the reaction non-spontaneous.
- Endothermic Reactions (ΔH>0) and Positive Entropy Change (ΔS>0):
- Spontaneous at High Temperatures: These reactions are non-spontaneous at low temperatures where the positive ΔH term dominates. At higher temperatures, the −TΔS term becomes more negative, making ΔG negative and the reaction spontaneous.
- Endothermic Reactions (ΔH>0) and Negative Entropy Change (ΔS<0):
- Never Spontaneous: These reactions are non-spontaneous at all temperatures because both terms (ΔH and −TΔS) contribute to making ΔG positive.