Learning Objectives
For the AP Chemistry exam, you should understand and be able to calculate heat transfer using specific heat capacity, heat capacity, and molar heat capacity. Master the use of calorimetry to measure heat changes in chemical reactions and physical processes, distinguishing between coffee cup and bomb calorimeters. Apply the principle of conservation of energy in calorimetry problems, and accurately perform calculations involving temperature changes, mass, and heat energy. Develop proficiency in solving problems that involve heat exchange between systems and their surroundings, and interpret experimental data from calorimetric experiments to determine enthalpy changes.
Free AP Chemistry Practice Test
Introduction
Heat capacity is a fundamental property of matter that measures the amount of heat required to change a substance's temperature by a certain amount. This concept is pivotal in understanding how different materials respond to thermal energy. Calorimetry, on the other hand, is the experimental technique used to measure the heat exchanged during physical and chemical processes. By using calorimeters, scientists can determine the heat capacity of substances, study reaction energetics, and explore thermodynamic properties, thereby gaining valuable insights into energy transfer and conservation in various system
Heat Capacity
What is Heat Capacity?
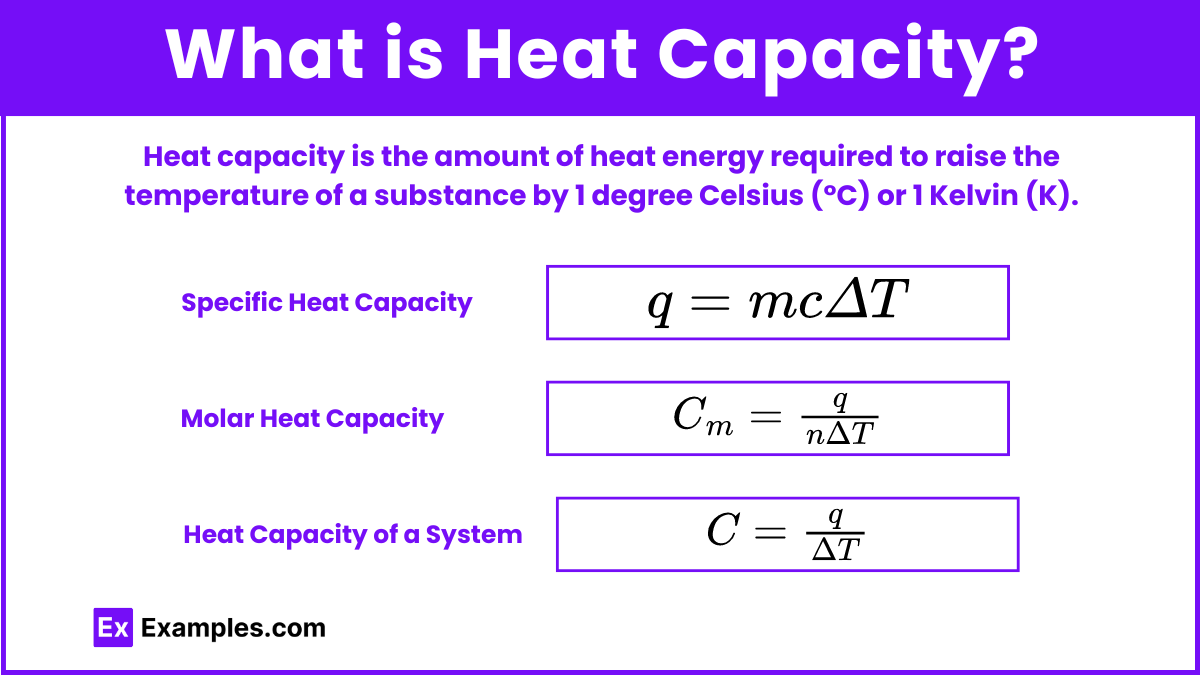
Heat capacity is the amount of heat energy required to raise the temperature of a substance by 1 degree Celsius (°C) or 1 Kelvin (K). It is an extensive property, meaning it depends on the amount of the substance present. The unit of heat capacity is typically joules per degree Celsius (J/°C) or joules per Kelvin (J/K).
Specific Heat Capacity
Specific heat capacity (c) is the amount of heat energy required to raise the temperature of 1 gram of a substance by 1 degree Celsius (°C) or 1 Kelvin (K). It is an intensive property, meaning it does not depend on the amount of the substance present. The unit of specific heat capacity is joules per gram per degree Celsius (J/g°C) or joules per gram per Kelvin (J/gK).
Formula:
q = mcΔT
Where:
q = heat energy (in joules, J).
m = mass of the substance (in grams, g).
c = specific heat capacity (in J/g°C).
ΔT = change in temperature (in °C or K).
Molar Heat Capacity
Molar heat capacity is the amount of heat energy required to raise the temperature of one mole of a substance by 1 degree Celsius (°C) or 1 Kelvin (K). It is an intensive property, meaning it does not depend on the amount of substance but rather on the substance's intrinsic properties. The unit of molar heat capacity is typically joules per mole per degree Celsius (J/mol°C) or joules per mole per Kelvin (J/molK).
Formula
Cm = molar heat capacity (in J/mol°C)
q = heat energy (in joules, J)
n = number of moles
ΔT = change in temperature (in °C or K)
Heat Capacity of a System
Heat capacity of a system is the amount of heat energy required to raise the temperature of the entire system by 1 degree Celsius (°C) or 1 Kelvin (K). It is an extensive property, meaning it depends on the total amount of material in the system. The unit of heat capacity is typically joules per degree Celsius (J/°C) or joules per Kelvin (J/K).
Formula
C =
C = heat capacity of the system (in J/°C or J/K)
q= heat energy (in joules, J)
ΔT = change in temperature (in °C or K)
Calorimetry
What is Calorimetry?
Calorimetry is the science of measuring the amount of heat transferred to or from a substance during a chemical reaction, physical change, or heat transfer process. It involves using a device called a calorimeter to accurately measure the heat exchanged in these processes.
Types of Calorimetry
1. Coffee Cup Calorimetry
Coffee cup calorimetry measures heat transfer at constant pressure. It is typically used for reactions occurring in aqueous solutions. A simple, insulated container like a coffee cup serves as the calorimeter, making it easy and effective for measuring heat changes in solution-based reactions.
Example: Dissolving salt in water.
2. Bomb Calorimetry
Bomb calorimetry measures heat transfer at constant volume. It is used for combustion reactions and involves a strong, sealed container called a bomb, placed in a water bath. This setup ensures that the entire heat released by the reaction is absorbed by the water, allowing accurate calculation of the energy change.
Example: Combustion of a fuel sample.
Calorimetry Equations
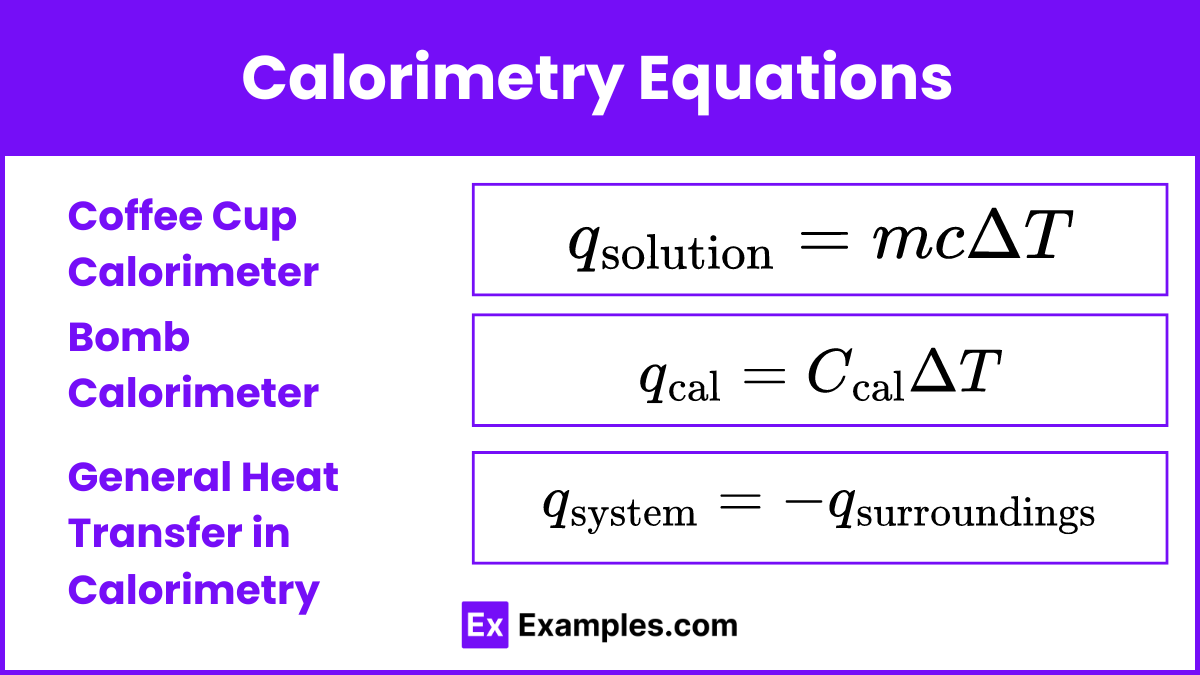
Coffee Cup Calorimeter (Constant Pressure)
For reactions in an aqueous solution using a coffee cup calorimeter, the heat absorbed or released by the solution can be calculated using the specific heat capacity formula:
Formula
= heat absorbed or released by the solution (in joules, J)
m = mass of the solution (in grams, g)
c = specific heat capacity of the solution (in J/g°C)
ΔT = change in temperature (in °C or K)
Bomb Calorimeter (Constant Volume)
For reactions in a bomb calorimeter, the heat absorbed or released by the calorimeter system can be calculated using the heat capacity of the calorimeter:
Formula
= heat absorbed or released by the calorimeter (in joules, J)
= heat capacity of the calorimeter (in J/°C or J/K)
ΔT = change in temperature (in °C or K)
General Heat Transfer in Calorimetry
In calorimetry, the principle of conservation of energy states that the heat gained or lost by the system is equal to the heat lost or gained by the surroundings.
Formula
This equation emphasizes that the total heat exchange in the system and its surroundings must sum to zero, indicating the conservation of energy.
Example Problem
Calculate the heat absorbed by 100 grams of water when its temperature increases from 25°C to 35°C. The specific heat capacity of water is 4.18 J/g°C.
Solution
Identify the given information:
Mass of water (mmm): 100 g
Initial temperature (Tᵢ): 25°C
Final temperature (Tբ): 35°C
Specific heat capacity of water (ccc): 4.18 J/g°C
Calculate the change in temperature (ΔT):
ΔT=Tբ − Tᵢ
ΔT = 35°C − 25°C
ΔT=10°C
Use the formula for heat transfer:
q = mcΔT
q = (100 g) (4.18 J/g°C) (10 °C)
Calculate the heat absorbed (q):
q = 100 × 4.18 × 10
q=4180 J
The heat absorbed by the water is 4180 joules (J).