Leaning Objectives
For the AP Chemistry exam, you should focus on understanding the mechanisms of heat transfer (conduction, convection, and radiation), the mathematical equations governing these processes. Master the concept of thermal equilibrium, including the zeroth law of thermodynamics, and how to apply it to solve problems involving temperature and heat transfer. Gain proficiency in calorimetry, including using a calorimeter to measure heat changes and calculating specific heat capacities. Develop the ability to analyze and interpret data related to heat transfer and thermal equilibrium in various chemical and physical contexts.
Introduction
Heat transfer and thermal equilibrium are fundamental concepts in thermodynamics, pivotal in understanding how energy moves and balances in various systems. Heat transfer refers to the movement of thermal energy from one object or substance to another, occurring through conduction, convection, or radiation. This energy transfer continues until thermal equilibrium is achieved, a state where the interacting bodies reach a uniform temperature, eliminating any temperature gradient. These principles are essential in numerous applications, from everyday appliances to complex industrial processes, making them crucial for both scientific inquiry and practical engineering.
Heat Transfer
What is Heat Transfer?
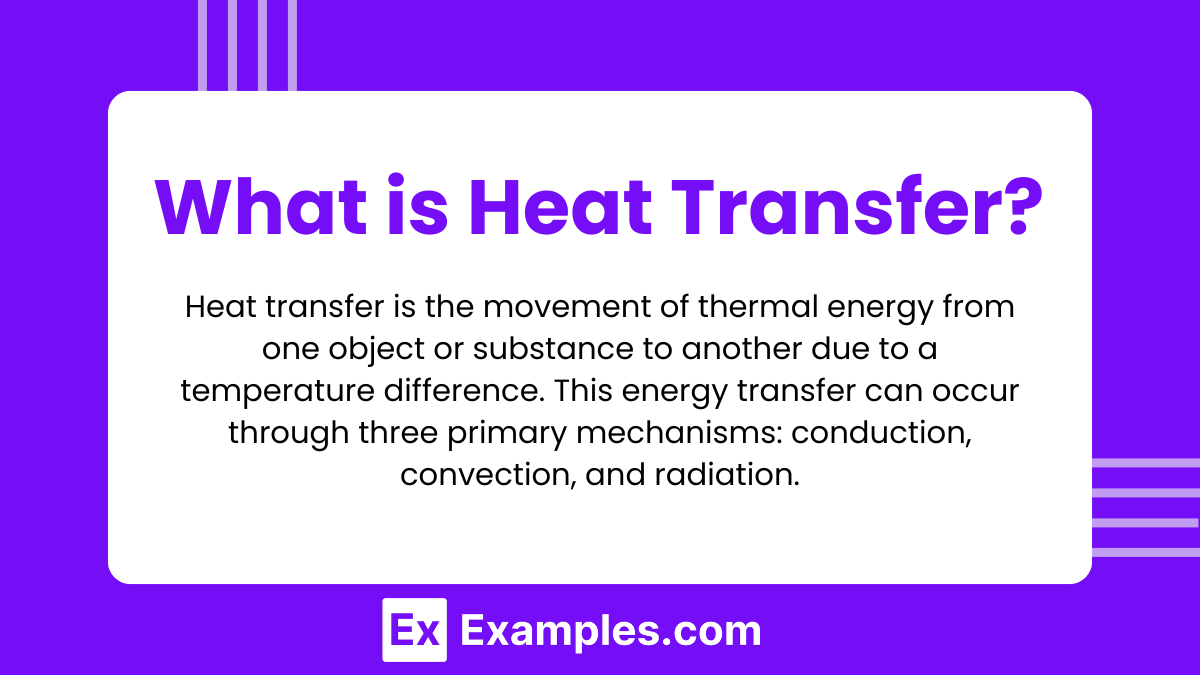
Heat transfer is the movement of thermal energy from one object or substance to another due to a temperature difference. This energy transfer can occur through three primary mechanisms: conduction, convection, and radiation.
Mechanisms of Heat Transfer
1. Conduction
Definition: Conduction is the transfer of heat through a material without any movement of the material itself. It occurs due to the direct interaction of particles within the material, such as atoms, molecules, or electrons.
How It Works:
- When one part of a material is heated, its particles vibrate more vigorously.
- These vibrations are passed to neighboring particles, transferring energy through the material.
- This process continues until thermal equilibrium is reached, with heat flowing from the hotter region to the cooler region.
Example: Heating one end of a metal rod. The heat travels along the rod through the vibrations of its atoms and free electrons.
2. Convection
Definition: Convection is the transfer of heat by the physical movement of a fluid (liquid or gas). It occurs when a warmer area of the fluid rises and is replaced by a cooler area of the fluid.
How It Works:
- When a fluid is heated, it becomes less dense and rises.
- The cooler, denser fluid then moves in to take its place, creating a circulation pattern.
- This cycle transfers heat through the fluid, distributing thermal energy.
Example: Boiling water in a pot. The hot water from the bottom rises to the top, while the cooler water descends, creating convection currents.
3. Radiation
Definition: Radiation is the transfer of heat through electromagnetic waves. It does not require a medium and can occur in a vacuum.
How It Works:
- All objects emit electromagnetic radiation based on their temperature.
- This radiation travels through space and can be absorbed by other objects, transferring energy.
- The amount and type of radiation depend on the temperature and nature of the emitting surface.
Example: The heat from the Sun reaching Earth. This heat travels through the vacuum of space via electromagnetic waves (primarily in the form of visible light and infrared radiation).
Mathematical Representation of Heat Transfer
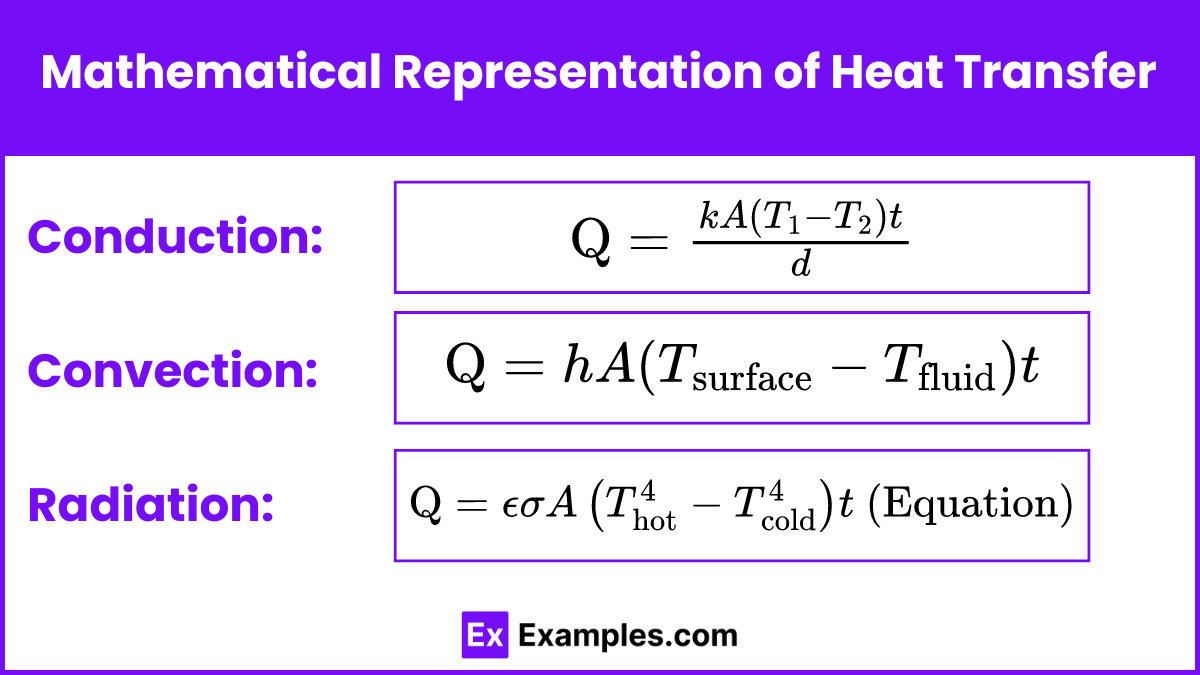
Conduction
The rate of heat transfer by conduction is given by Fourier’s law:
![]()
where:
- Q is the heat transferred (Joules, J)
- k is the thermal conductivity of the material (W/m·K)
- A is the cross-sectional area through which heat is transferred (m²)
- T₁ and T₂ are the temperatures at the two ends of the material (Kelvin, K or Celsius, °C)
- t is the time during which the heat transfer occurs (seconds, s)
- d is the thickness of the material (meters, m)
Convection
The rate of heat transfer by convection is described by Newton’s law of cooling:
![]()
where:
- Q is the heat transferred (Joules, J)
- h is the convective heat transfer coefficient (W/m²·K)
- A is the surface area through which heat is transferred (m²)
- TSurface is the temperature of the surface (Kelvin, K or Celsius, °C)
- Tfluid is the temperature of the fluid (Kelvin, K or Celsius, °C)
- t is the time during which the heat transfer occurs (seconds, s)
Radiation
The rate of heat transfer by radiation is given by the Stefan-Boltzmann law:
![]()
where:
- Q is the heat transferred (Joules, J)
- ϵ is the emissivity of the material (dimensionless, ranges from 0 to 1)
- σ is the Stefan-Boltzmann constant (5.67×10⁻⁸ W/m².K⁴)
- A is the surface area through which heat is transferred (m²)
- Thot is the absolute temperature of the hotter body (Kelvin, K)
- Tcold is the absolute temperature of the cooler body (Kelvin, K)
- t is the time during which the heat transfer occurs (seconds, s)
Examples of Heat Transfer
Conduction
- A metal spoon getting hot when its handle is left in a pot of boiling water.
- Heat traveling through a copper wire from one end to the other.
- Touching a hot stove and feeling the heat transfer to your hand.
- Heating one end of an iron rod and observing the other end becoming hot after some time.
- A hot coffee mug warming the surface of a wooden table it’s placed on.
Convection
- Boiling water in a pot, where hot water rises and cooler water descends, creating convection currents.
- Warm air rising and cool air sinking in a room, causing a convection cycle.
- The circulation of air in the atmosphere, which helps distribute heat around the Earth.
- Heating a room with a radiator, where warm air rises and cool air moves in to replace it.
- Ocean currents transferring warm water from the equator to the poles.
Radiation
- The Sun warming the Earth through the vacuum of space.
- Feeling the heat from a campfire even when you’re sitting several feet away.
- The warmth you feel from a heat lamp or infrared heater.
- A microwave oven heating food by emitting microwaves.
- The heat radiating from a hot asphalt road on a sunny day.
Thermal Equilibrium
What is Thermal Equilibrium?
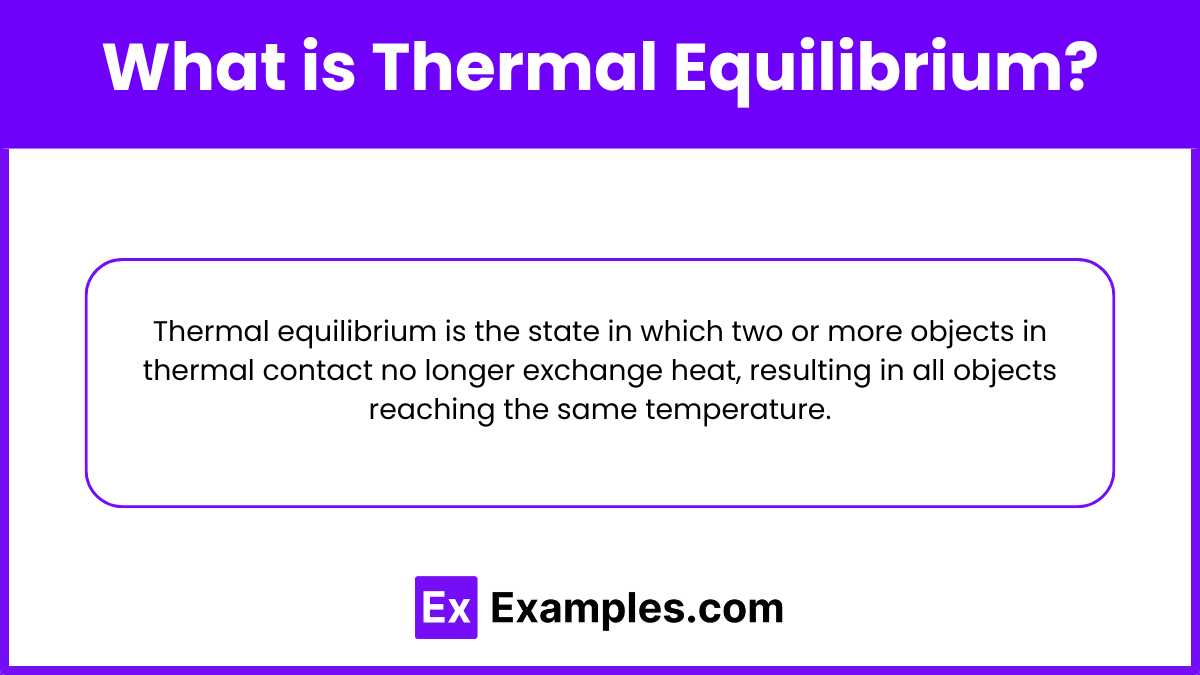
Thermal equilibrium is the state in which two or more objects in thermal contact no longer exchange heat, resulting in all objects reaching the same temperature.
Principles of Thermal Equilibrium
Zeroth Law of Thermodynamics
Statement: If two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
Explanation: This principle establishes temperature as a fundamental and measurable property. If object A is in thermal equilibrium with object B, and object B is in thermal equilibrium with object C, then object A is also in thermal equilibrium with object C. This law allows the use of thermometers as a means to measure temperature accurately.
Temperature Equalization
Principle: When two objects at different temperatures come into thermal contact, heat will flow from the hotter object to the cooler object until both objects reach the same temperature. This process continues until there is no net flow of heat between the objects, achieving thermal equilibrium.
Conservation of Energy
Principle: In an isolated system, the total energy remains constant. When heat flows between objects within the system, the heat lost by the hotter object is equal to the heat gained by the cooler object. This ensures that energy is conserved during the process of reaching thermal equilibrium.
Mathematical Representation of Thermal Equilibrium
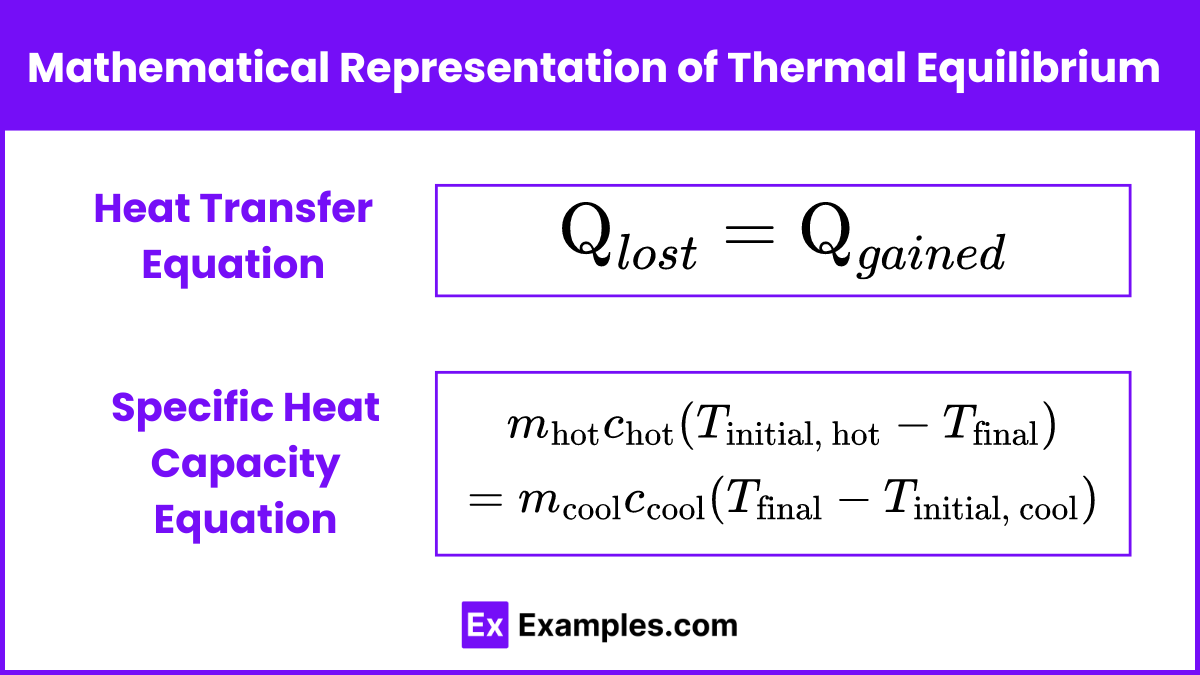
The mathematical representation of thermal equilibrium involves the principle of conservation of energy, particularly the concept that the heat lost by a hotter object is equal to the heat gained by a cooler object. Here’s how it can be represented:
Heat Transfer Equation
When two objects with different initial temperatures come into thermal contact and reach thermal equilibrium, the heat lost by the hot object![]() equals the heat gained by the cold object
equals the heat gained by the cold object ![]() :
:![]()
Specific Heat Capacity Equation
For a hot object and a cool object, the heat transfer can be expressed using their specific heat capacities (c):![]()
where:
- m is the mass of the object (kg)
- c is the specific heat capacity of the material (J/kg·K)
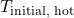 is the initial temperature of the hot object (K or °C)
is the initial temperature of the hot object (K or °C)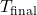 is the final equilibrium temperature (K or °C)
is the final equilibrium temperature (K or °C)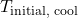 is the initial temperature of the cool object (K or °C)
is the initial temperature of the cool object (K or °C)
Examples of Thermal Equilibrium
- Metal Spoon in Hot Soup: When a metal spoon is placed in a bowl of hot soup, the spoon initially at room temperature gains heat from the soup until both the spoon and the soup reach the same temperature, achieving thermal equilibrium.
- Ice in a Drink: Adding ice cubes to a warm drink will cause the ice to melt as it absorbs heat from the drink. Eventually, the drink and the melted ice (now water) will reach the same temperature, resulting in thermal equilibrium.
- Body Temperature: When a person steps into a cold room, their body will lose heat to the surrounding air. After a period of time, the person’s body and the room air will reach the same temperature, assuming no external heat sources, achieving thermal equilibrium.
- Refrigerated Food: A hot dish placed in a refrigerator will lose heat to the cooler air inside the refrigerator. Over time, the dish and the refrigerator air will reach the same temperature, resulting in thermal equilibrium.
- Thermometer in Liquid: When a thermometer is placed in a cup of hot water, the mercury or alcohol inside the thermometer will expand until it reaches the same temperature as the water. The thermometer and the water are then in thermal equilibrium.