Learning Objectives
By studying Hess's Law for the AP Chemistry exam, you should be able to understand and apply the principle that the total enthalpy change of a reaction is independent of the pathway taken. You should be able to perform calculations using Hess's Law to determine the enthalpy changes of reactions by combining known enthalpies of formation or reaction steps. Additionally, you should be adept at manipulating and reversing thermochemical equations, understanding the concept of state functions, and utilizing Hess's Law in various problem-solving scenarios to predict energy changes in chemical reactions accurately.
Free AP Chemistry Practice Test
Introduction
Hess's law, a fundamental principle in thermochemistry, states that the total enthalpy change of a chemical reaction is the same, regardless of the pathway taken, provided the initial and final states are identical. This law is based on the conservation of energy and allows chemists to determine enthalpy changes for complex reactions by breaking them down into simpler steps with known enthalpy changes. By applying Hess's law, one can calculate the enthalpy change of reactions that are difficult to measure directly, facilitating the study and application of thermodynamic principles in various chemical processes.
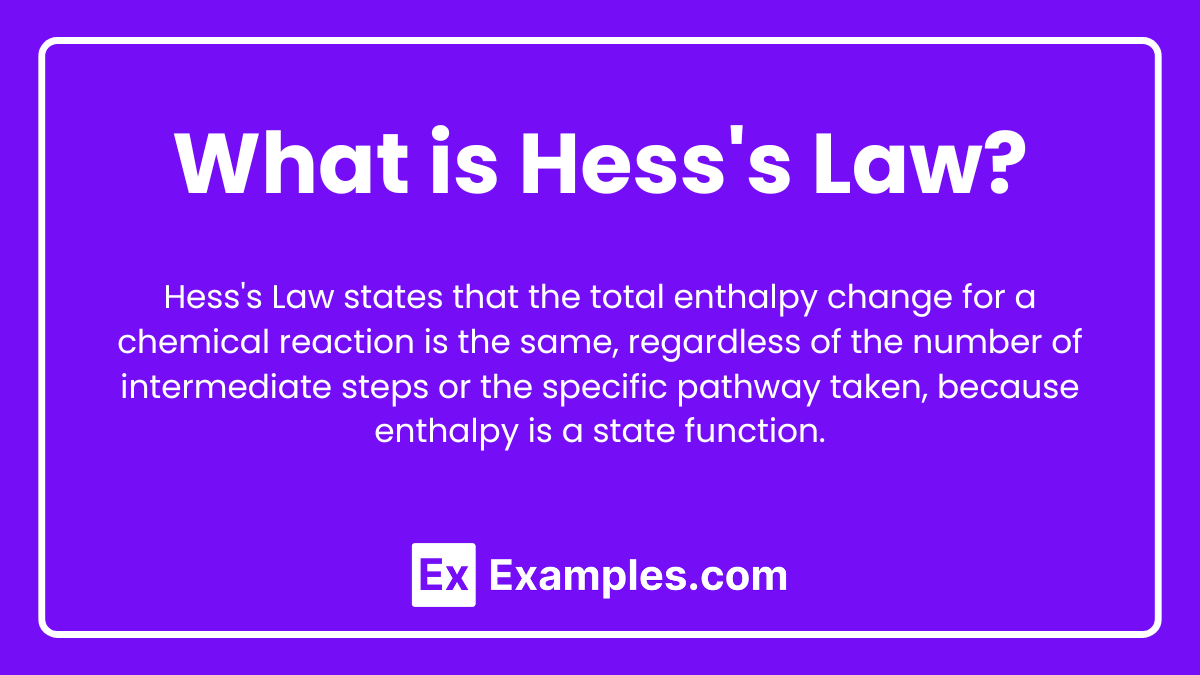
What is Hess's Law?
Hess's Law states that the total enthalpy change for a chemical reaction is the same, regardless of the number of intermediate steps or the specific pathway taken, because enthalpy is a state function.
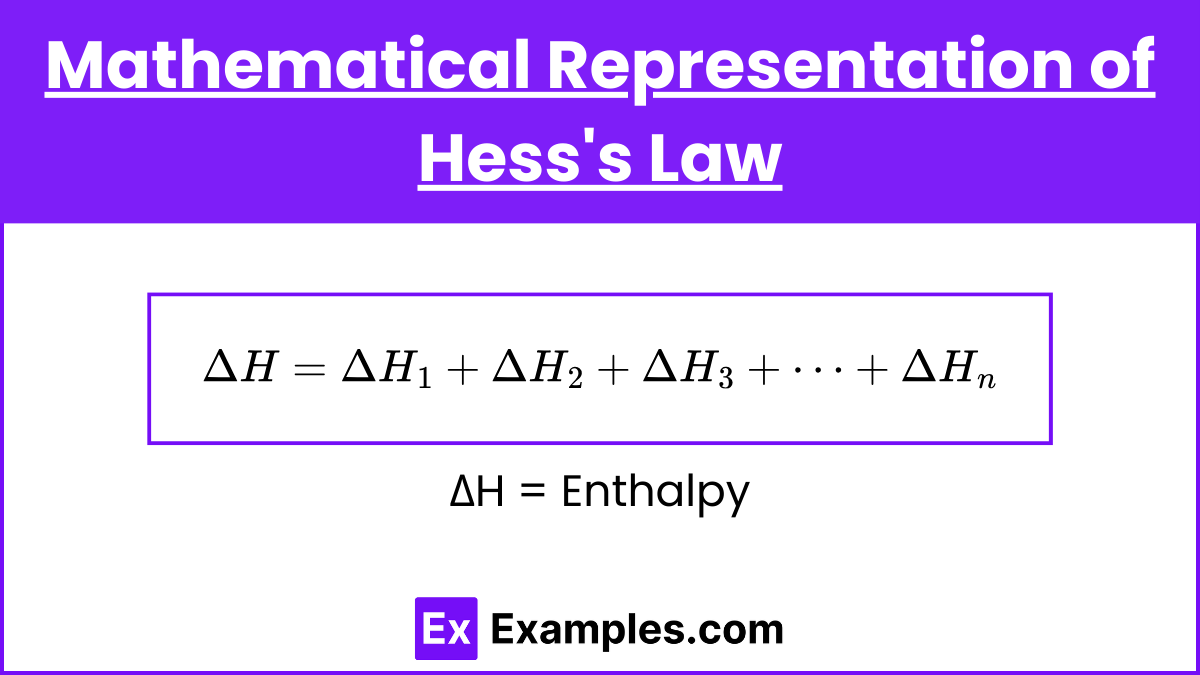
Mathematical Representation of Hess's Law
Hess's Law can be represented mathematically by considering a series of reactions that sum to an overall reaction. If a reaction can be expressed as the sum of two or more reactions, the enthalpy change for the overall reaction is the sum of the enthalpy changes for the individual reactions.
If:
Then:
This can be extended to any number of intermediate steps:
Key Concepts of Hess’s law
Enthalpy (H)
Definition: Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy of the system plus the product of the system's pressure and volume.
Symbol: H
Units: Joules (J) or kilojoules (kJ)
Significance: Enthalpy change (ΔH) indicates the heat absorbed or released during a chemical reaction at constant pressure. An exothermic reaction releases heat (ΔH<0), while an endothermic reaction absorbs heat (ΔH>0).
State Function
Definition: A state function is a property of a system that depends only on the current state of the system, not on the path taken to reach that state.
Examples: Enthalpy (H), entropy (S), and free energy (G).
Significance: Because enthalpy is a state function, the change in enthalpy for a reaction depends only on the initial and final states, not on the intermediate steps. This is the foundational principle behind Hess's Law.
Thermochemical Equations
Definition: Thermochemical equations are balanced chemical equations that include the enthalpy change of the reaction.
Format: These equations show the reactants and products of a reaction, along with the enthalpy change (ΔH). For example: C(s) + O₂(g) → CO₂(g) ΔH = −393.5 kJ
Significance: Thermochemical equations allow us to quantitatively describe the energy changes in chemical reactions. They are essential for applying Hess's Law, as they provide the necessary enthalpy changes for individual steps in a reaction pathway.
Reaction Pathway
Definition: The reaction pathway is the series of steps or intermediate reactions that occur from the reactants to the products.
Significance: According to Hess's Law, the enthalpy change of the overall reaction is independent of the reaction pathway. This means that no matter how many steps or intermediate reactions are involved, the total enthalpy change remains the same.
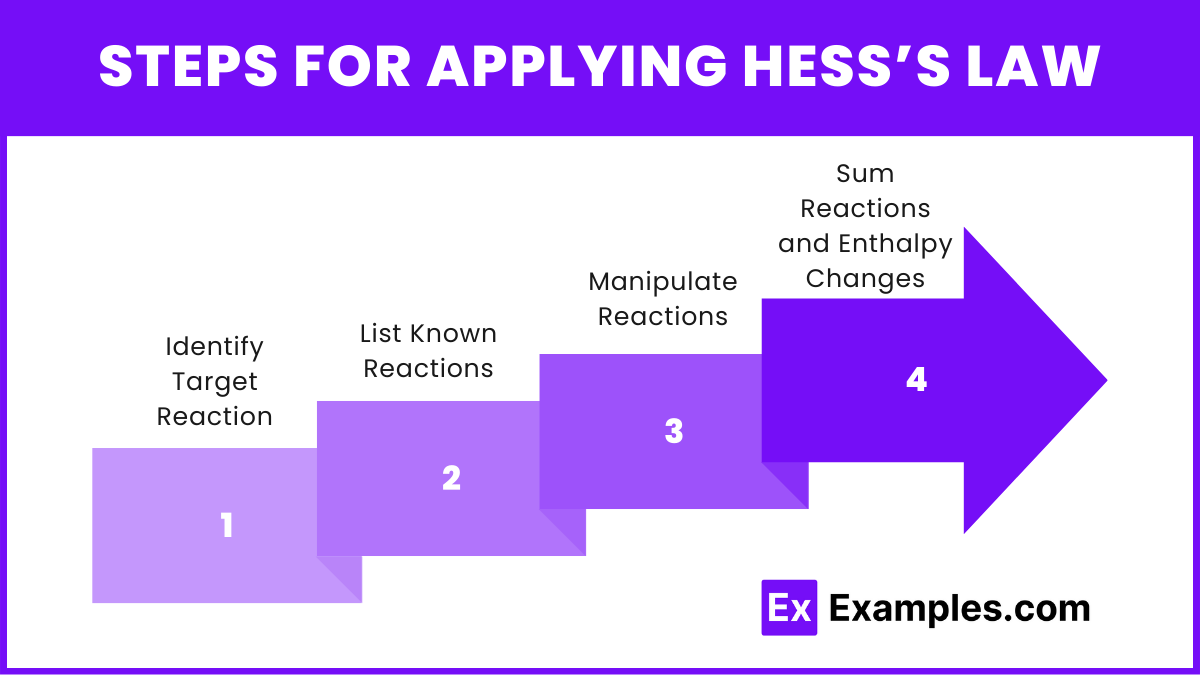
Steps for Applying Hess’s Law
Identify Target Reaction: Determine the overall reaction for which you need to find the enthalpy change.
List Known Reactions: Write down the given reactions with their enthalpy changes (ΔH\Delta HΔH).
Manipulate Reactions: Adjust the given reactions by reversing them or multiplying by coefficients to match the target reaction.
Sum Reactions and Enthalpy Changes: Add the adjusted reactions and their enthalpy changes to obtain the target reaction and its total enthalpy change.
Applications of Hess's Law
Calculating Enthalpy Changes: Determine the enthalpy change of a reaction by combining known enthalpy changes of related reactions.
Determining Heats of Formation: Use Hess's Law to find the enthalpy changes for the formation of compounds from their elements.
Thermochemical Equations: Balance and calculate the enthalpy changes for complex reactions using simpler known reactions.
Predicting Reaction Feasibility: Assess the energy requirements or releases to predict whether a reaction is likely to occur under given conditions.