Learning Objectives
For the AP Chemistry exam, you should learn to identify and explain the different types of intermolecular forces (London dispersion forces, dipole-dipole interactions, hydrogen bonding, and ion-dipole interactions), understand the factors that affect the strength of these forces, and describe how intermolecular forces influence physical properties such as boiling and melting points, viscosity, surface tension, and solubility. You should also be able to predict the relative strengths of intermolecular forces in different substances, explain how molecular size and polarity affect intermolecular interactions, and apply the “like dissolves like” principle to solubility scenarios.
Introduction
Intermolecular forces (IMFs) are the forces of attraction or repulsion between neighboring molecules, atoms, or ions, significantly influencing the physical properties of substances. These forces are distinct from intramolecular forces, which are the bonds within a molecule holding atoms together, such as covalent or ionic bonds. While intramolecular forces determine the chemical properties of a substance, intermolecular forces primarily affect its physical characteristics, including boiling points, melting points, solubility, and viscosity. Understanding IMFs is crucial for predicting and explaining the behavior of substances in various chemical contexts
What are Intermolecular Forces?
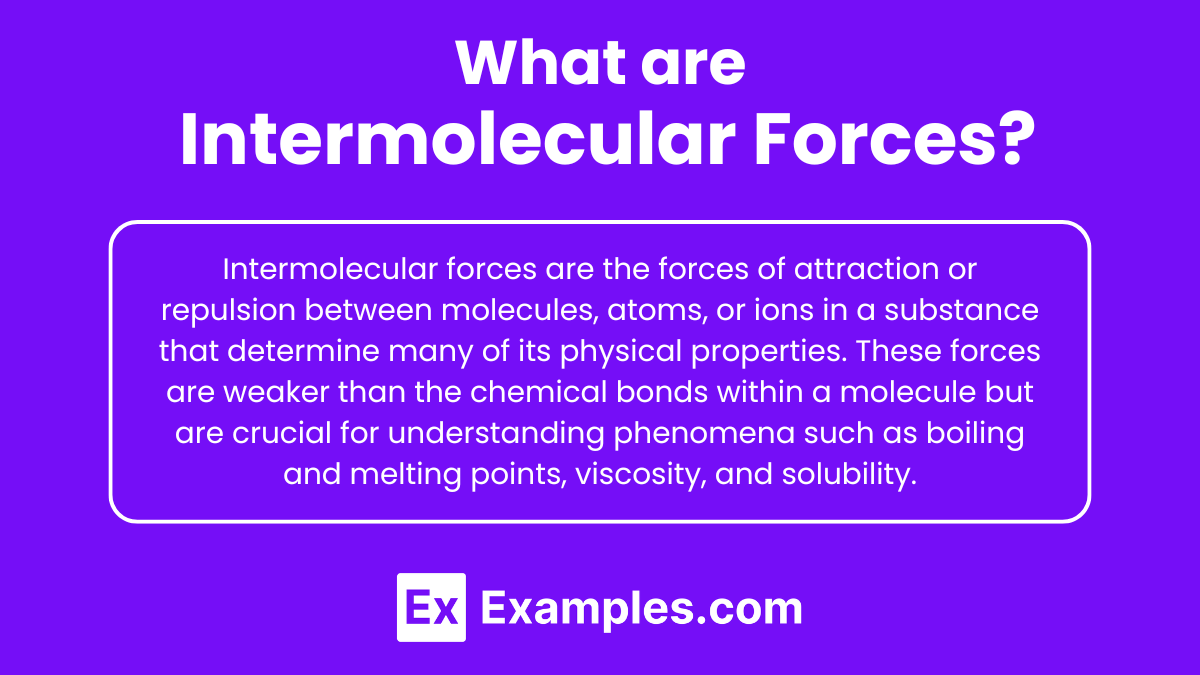
Intermolecular forces are the forces of attraction or repulsion between molecules, atoms, or ions in a substance that determine many of its physical properties. These forces are weaker than the chemical bonds (intramolecular forces) within a molecule but are crucial for understanding phenomena such as boiling and melting points, viscosity, and solubility.
Types of Intermolecular Forces
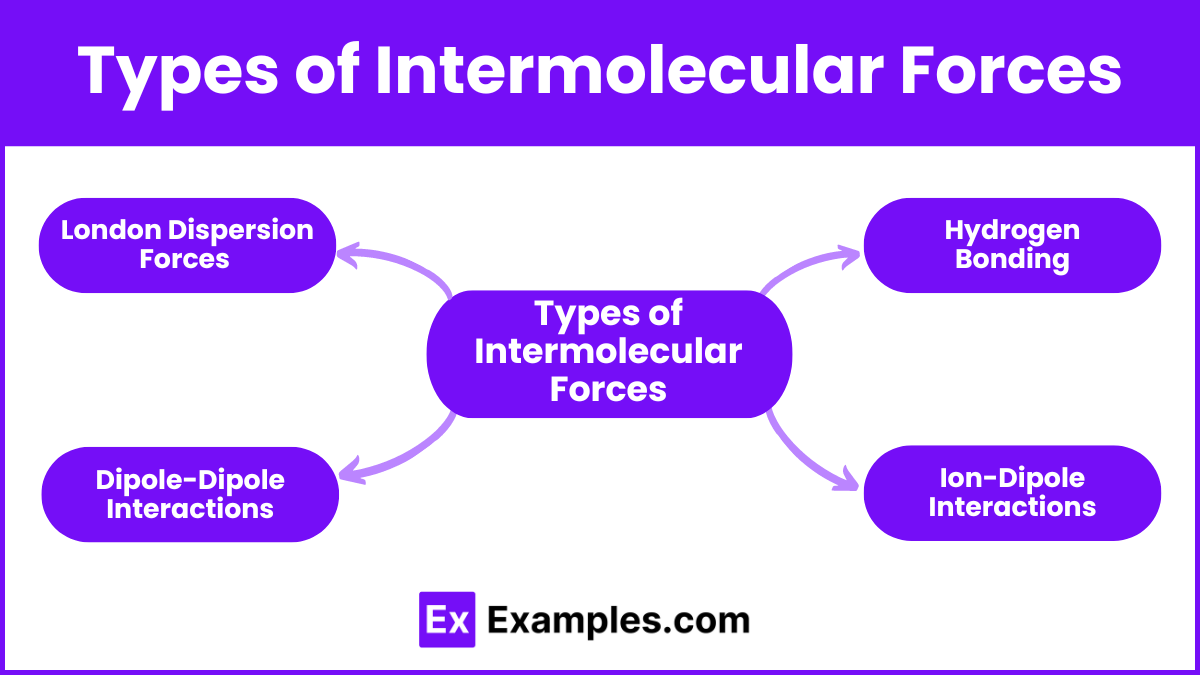
1. London Dispersion Forces (LDFs)
- Description: London dispersion forces are weak, temporary attractive forces that arise from the momentary distribution of electrons around atoms or molecules, creating temporary dipoles. These forces are present in all molecules, whether polar or nonpolar.
- Example: In helium (He) gas, LDFs are the primary intermolecular force, causing slight attractions between helium atoms.
2. Dipole-Dipole Interactions
- Description: Dipole-dipole interactions occur between the positive end of one polar molecule and the negative end of another. These forces are stronger than London dispersion forces but weaker than hydrogen bonds.
- Example: In hydrogen chloride (HCl), the positive end of the HCl molecule (hydrogen) is attracted to the negative end (chlorine) of another HCl molecule.
3. Hydrogen Bonding
- Description: Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) is attracted to an electronegative atom in a nearby molecule. This is one of the strongest intermolecular forces.
- Example: In water (H₂O), the hydrogen atoms of one water molecule form hydrogen bonds with the oxygen atom of another water molecule, leading to water’s high boiling point.
4. Ion-Dipole Interactions
- Description: Ion-dipole interactions are the forces of attraction between an ion and a polar molecule. These interactions are significant in solutions where ionic compounds are dissolved in polar solvents.
- Example: When sodium chloride (NaCl) dissolves in water, the sodium ions (Na⁺) are attracted to the negative end (oxygen) of water molecules, and the chloride ions (Cl⁻) are attracted to the positive end (hydrogen) of water molecules.
Factors Affecting Intermolecular Forces
- Molecular Size and Shape: Larger molecules with more electrons have stronger London dispersion forces; linear molecules have stronger dispersion forces than branched ones.
- Polarity: Polar molecules exhibit stronger dipole-dipole interactions; the greater the polarity, the stronger the interactions.
- Hydrogen Bonding: Molecules with hydrogen atoms bonded to N, O, or F show strong hydrogen bonding, enhancing intermolecular forces.
- Ion Presence: Ion-dipole interactions in solutions with ionic compounds are stronger than other intermolecular forces.
- Molecular Symmetry: Symmetrical molecules have weaker dipole-dipole interactions, while asymmetrical molecules have stronger ones.
Physical Properties Influenced by Intermolecular Forces(IMFs)
- Boiling and Melting Points: Stronger IMFs lead to higher boiling and melting points (e.g., water has a high boiling point due to hydrogen bonding).
- Viscosity: Substances with stronger IMFs have higher viscosity (e.g., glycerol has high viscosity due to hydrogen bonding).
- Surface Tension: Strong IMFs result in higher surface tension (e.g., water has high surface tension due to hydrogen bonding).
- Solubility: “Like dissolves like” principle, where similar IMFs enhance solubility (e.g., NaCl dissolves in water due to ion-dipole interactions).
- Capillary Action: Influenced by cohesive and adhesive forces, affecting liquid flow in narrow spaces (e.g., water rises in thin glass tubes due to hydrogen bonding).
- Vapor Pressure: Weaker IMFs result in higher vapor pressures (e.g., ether has higher vapor pressure than water due to weaker London dispersion forces).
- Heat of Vaporization: Higher for substances with stronger IMFs (e.g., water has a high heat of vaporization due to strong hydrogen bonding).
Comparing Intermolecular Forces
| Type of IMF | Strength | Example | Occurrence |
|---|---|---|---|
| London Dispersion Forces | Weakest | Argon (Ar), methane (CH₄) | Present in all molecules, stronger in larger and more massive molecules |
| Dipole-Dipole Interactions | Moderate | Hydrogen chloride (HCl) | Only in polar molecules |
| Hydrogen Bonding | Strong | Water (H₂O), ammonia (NH₃) | When H is bonded to N, O, or F |
| Ion-Dipole Interactions | Strongest | Sodium chloride (NaCl) in water | Between ions and polar molecules |
Examples
- Methane (CH₄): Nonpolar molecules exhibit weak, temporary dipoles, leading to London dispersion forces.
- Iodine (I₂): A larger nonpolar molecule with stronger London dispersion forces due to increased electron cloud.
- Acetone (CH₃COCH₃): Polar molecule with a permanent dipole moment leading to dipole-dipole attractions.
- Ethanol (C₂H₅OH): Presence of an -OH group bonded to hydrogen, allowing strong hydrogen bonding.
- Magnesium chloride (MgCl₂) in water (H₂O): Mg²⁺ and Cl⁻ ions interact strongly with the polar water molecules.