Learning Objectives
Understanding chemical reactions is essential for excelling in AP Chemistry. You should be able to define chemical reactions, identify key components like reactants, products, and coefficients, and classify different types of reactions, including synthesis, decomposition, single replacement, double replacement, combustion, and redox reactions. Mastery of predicting reaction products using patterns and rules, applying the activity series for single replacement reactions, and using solubility rules for double replacement reactions is crucial. Additionally, balancing chemical equations to ensure mass conservation, distinguishing between exothermic and endothermic reactions, and understanding catalysts’ roles are key learning objectives.
Introduction
Chemical reactions occur when reactants undergo changes to form products with different properties. These reactions can be classified into several types, including synthesis, decomposition, single replacement, double replacement, combustion, and redox reactions, each with distinct characteristics and patterns. By mastering these concepts, you can predict the outcomes of reactions, balance chemical equations, and understand the energy changes involved.
What are Chemical Reactions?
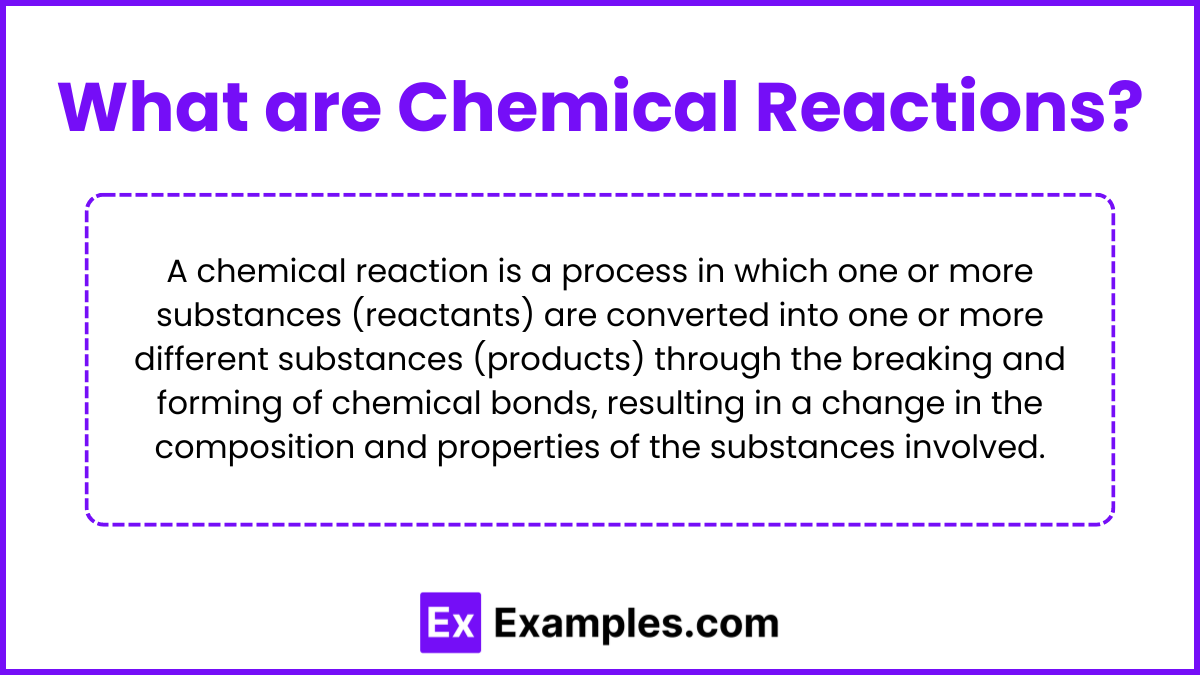
A chemical reaction is a process in which one or more substances (reactants) are converted into one or more different substances (products) through the breaking and forming of chemical bonds, resulting in a change in the composition and properties of the substances involved.
Key Components of a Chemical Reaction
- Reactants: Substances that start the reaction and undergo change. They are present on the left side of the chemical equation.
- Products: New substances formed as a result of the reaction. They are found on the right side of the chemical equation.
- Coefficients: Numbers placed before the chemical formulas to indicate the number of molecules or atoms of each substance involved in the reaction. They help balance the equation to ensure the conservation of mass.
- Subscripts: Small numbers written within chemical formulas to show the number of atoms of each element in a molecule.
- States of Matter: Symbols indicating the physical state of the substances involved:
- s for solid
- l for liquid
- g for gas
- aq for aqueous (dissolved in water)
- Yield Arrow (→): Indicates the direction of the reaction, showing the conversion of reactants to products. It can also be used with double arrows (⇌) to show reversible reactions.
- Catalysts: Substances that speed up the reaction without being consumed. They are often indicated above or below the yield arrow.
- Reaction Conditions: Information such as temperature, pressure, or the presence of a catalyst that affects the reaction. These are often written above or below the yield arrow.
Types of Chemical Reactions
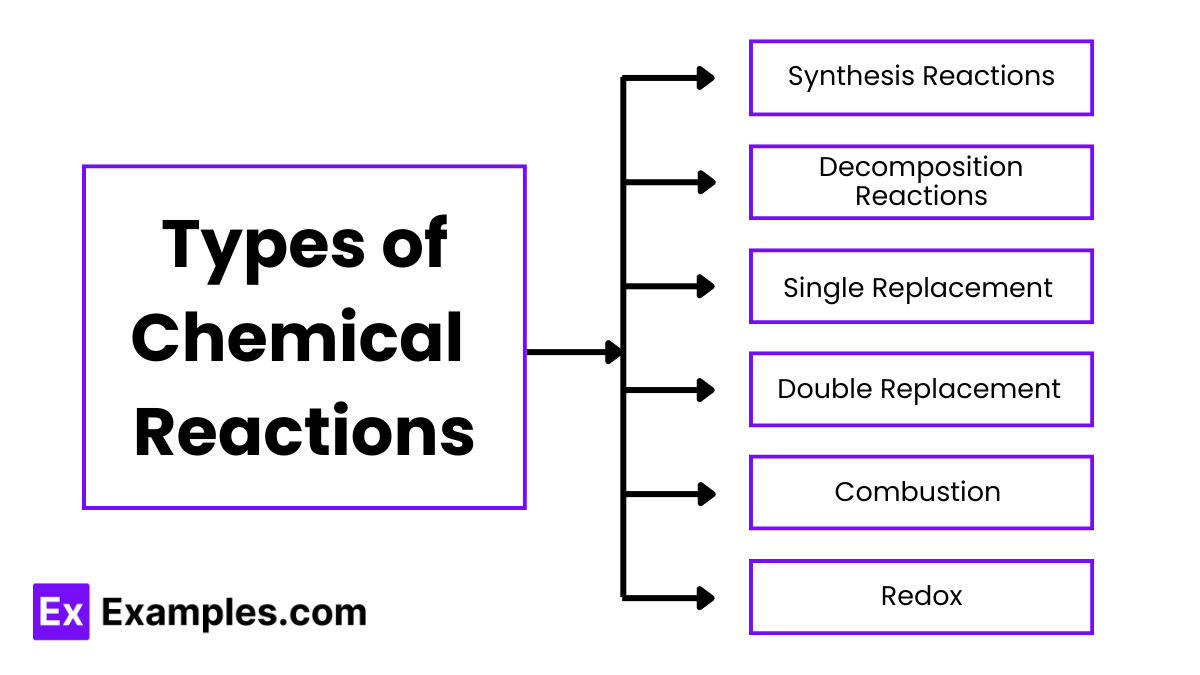
1. Synthesis Reactions (Combination Reactions)
In a synthesis reaction, two or more simple substances combine to form a more complex product.
General Form: A + B → AB
Example: 2H₂ + O₂ → 2H₂O
2. Decomposition Reactions
In a decomposition reaction, a single compound breaks down into two or more simpler substances.
General Form: AB → A + B
Example: 2H₂O₂ → 2H₂O + O₂
3. Single Replacement Reactions
In a single replacement reaction, one element replaces another element in a compound.
General Form: A + BC → AC + B
Example: Zn + 2HCl → ZnCl₂ + H₂
4. Double Replacement Reactions
In a double replacement reaction, the ions of two compounds exchange places to form two new compounds.
General Form: AB + CD → AD + CB
Example: Na₂SO₄ + BaCl₂ → BaSO₄ +2NaCl
5. Combustion Reactions
In a combustion reaction, a substance reacts with oxygen to produce energy in the form of heat and light, usually producing carbon dioxide and water if a hydrocarbon is involved.
General Form: Hydrocarbon + O₂ → CO₂ + H₂O
Example: CH₄ + 2O₂ → CO₂ + 2H₂O
6. Redox Reactions (Oxidation-Reduction Reactions)
Redox reactions involve the transfer of electrons between substances. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons.
Example: 2Na + Cl₂ → 2NaCl
Oxidation: Na → Na⁺ + e⁻
Reduction: Cl₂ + 2e⁻ → 2Cl
Identifying Reaction Types
Synthesis (Combination) Reactions
- Characteristics: Two or more reactants combine to form a single product.
- Clues: Look for multiple reactants forming one product.
Decomposition Reactions
- Characteristics: A single reactant breaks down into two or more products.
- Clues: One reactant forming multiple products.
Single Replacement Reactions
- Characteristics: One element replaces another element in a compound.
- Clues: An element and a compound react to form a different element and a different compound.
Double Replacement Reactions
- Characteristics: The ions of two compounds exchange places to form two new compounds.
- Clues: Two compounds react to form two different compounds, often resulting in a precipitate, gas, or water.
Combustion Reactions
- Characteristics: A substance reacts with oxygen, releasing energy in the form of heat and light, and typically producing carbon dioxide and water.
- Clues: A hydrocarbon or other organic compound reacts with oxygen.
Redox (Oxidation-Reduction) Reactions
- Characteristics: Involves the transfer of electrons between reactants, resulting in changes in oxidation states.
- Clues: Look for changes in the oxidation numbers of elements in the reactants and products.
Using Activity Series for Single Replacement Reactions
- Activity Series: A list of elements ordered by their reactivity. In single replacement reactions, a more reactive element will replace a less reactive element in a compound.
- Example:
Solubility Rules for Double Replacement Reactions
- Solubility Rules: Guidelines that predict whether a precipitate will form in a double replacement reaction.
- Example:
- Na₂CO₃(aq) + CaCl₂(aq) → CaCO₃(s) + 2NaCl(aq)
- Calcium carbonate (CaCO₃) is insoluble and forms a precipitate.
Balancing Chemical Equations
Balancing chemical equations ensures that the same number of each type of atom is present on both sides of the equation, following the Law of Conservation of Mass. Here’s a step-by-step guide to balancing chemical equations:
Steps to Balance a Chemical Equation
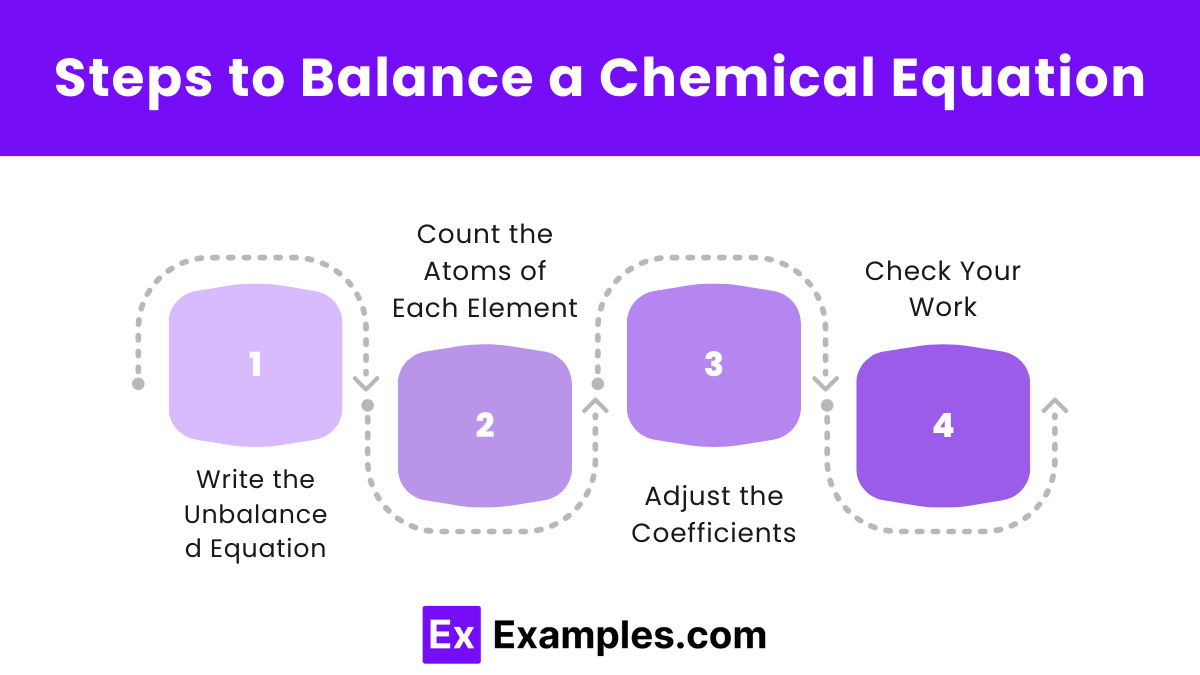
- Write the Unbalanced Equation: Start with the correct formulas for all reactants and products. Example: C₃H₃ ₈ + O₂ → CO₂ + H₂O
- Count the Atoms of Each Element: List the number of atoms for each element present in the reactants and products. Reactants:
- Carbon (C): 3Hydrogen (H): 8Oxygen (O): 2
- Carbon (C): 1
- Hydrogen (H): 2
- Oxygen (O): 3 (2 from CO₂ and 1 from H_2O)
- Adjust the Coefficients: Begin by balancing the elements that appear in only one reactant and one product.
- Balance Carbon (C): C₃H₈ + O₂ → 3CO₂ + H₂O
- Balance Hydrogen (H): C₃H₈ + O₂→ 3CO₂+ 4H₂O
- Balance Oxygen (O): Oxygen appears in both CO₂ and H₂O on the product side.
- Total Oxygen atoms needed: 3×2+4×1=6+4=10
- Check Your Work: Confirm that the number of atoms of each element is equal on both sides of the equation. Reactants:
- Carbon (C): 3 Hydrogen (H): 8 Oxygen (O): 10 (from 5 O₂ molecules)
- Carbon (C): 3 (from 3 CO₂ molecules)
- Hydrogen (H): 8 (from 4 H₂O molecules)
- Oxygen (O): 10 (6 from 3 CO₂ molecules and 4 from 4 H₂O molecules)
Example of a Balanced Equation
Unbalanced Equation: C₃H₈ + O₂ → CO₂ + H₂O
Balanced Equation: C₃H₈+5O₂ → 3CO₂ + 4H₂O
Tips for Balancing Equations
- Balance one element at a time: Start with the element that appears in the fewest compounds and work your way to the element that appears in the most.
- Leave hydrogen and oxygen for last: These elements are often found in multiple compounds, so balance them after other elements.
- Use the smallest whole-number coefficients: After balancing, ensure that all coefficients are in the simplest whole-number ratio.
- Double-check your work: Count the atoms for each element on both sides of the equation to verify that they are balanced.
Energy Changes in Reactions
Exothermic Reactions
Exothermic reactions release energy to the surroundings, usually in the form of heat or light. These reactions occur when the energy required to break the bonds in the reactants is less than the energy released when new bonds form in the products.
- Characteristics:
- Temperature of the surroundings increases.
- Energy is released as heat, light, or both.
- Products are typically more stable than reactants.
- Example:
- Combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O + energy
- Real-life Example:
- Burning wood in a fireplace releases heat and light.
Endothermic Reactions
Endothermic reactions absorb energy from the surroundings. These reactions occur when the energy required to break the bonds in the reactants is greater than the energy released when new bonds form in the products.
- Characteristics:
- Temperature of the surroundings decreases.
- Energy is absorbed from the surroundings, usually as heat.
- Products are typically less stable than reactants.
- Example:
- Decomposition of calcium carbonate: CaCO₃ → CaO + CO₂ (energy is absorbed to break down calcium carbonate)
- Real-life Example:
- Cooking an egg absorbs heat energy from the stove.
Energy Diagrams
Energy diagrams visually represent the energy changes during a reaction. They show the energy of the reactants and products, as well as the activation energy required to initiate the reaction.
- Exothermic Reaction Diagram:
- The reactants are higher in energy than the products.
- The difference in height between reactants and products represents the energy released.
- The activation energy is the peak of the curve, showing the energy needed to start the reaction.
- Endothermic Reaction Diagram:
- The reactants are lower in energy than the products.
- The difference in height between reactants and products represents the energy absorbed.
- The activation energy is the peak of the curve, showing the energy needed to start the reaction.
Activation Energy
Activation energy (Ea) is the minimum energy required for a reaction to occur. It represents the energy barrier that reactants must overcome to form products. Both exothermic and endothermic reactions require activation energy.
- Lower activation energy: Faster reaction rate.
- Higher activation energy: Slower reaction rate.
Catalysts
Catalysts are substances that increase the reaction rate without being consumed in the reaction. They work by lowering the activation energy, making it easier for the reaction to proceed.
- Example:
- Enzymes in the human body act as catalysts to speed up biochemical reactions.