Efficiently convert decimals to percentages using our Decimal to Percent Calculator at examples.com. Achieve fast and accurate results with our intuitive interface.
Decimal to Percent Calculator
Formula: Decimal to Percent Calculator : Percentage = Decimal * 100 %
Enter a Decimal :
Result: The percentage is: 5000%
How to Use Decimal to Percent Calculator
Step 1: Enter the Decimal
- Locate the input box labeled “Enter a Decimal:”.
- Type in the decimal number you want to convert to a percentage. For example, if you want to convert the number 50, just type “50” into this box.
Step 2: Calculate
- Click on the “Calculate” button. This will process the decimal value using the formula:
- Percentage=Decimal×100
- The calculator will then display the result in a new area or box, stating “The percentage is:,” followed by the calculated percentage value.
Step 3: Reset (if necessary)
- If you wish to perform another calculation, click the “Reset” button to clear the previous input and results, allowing you to start a new calculation with a different decimal value.
These steps will guide you through converting any decimal value into a percentage using the specified calculator, as shown in the screenshot you provided.
How to Calculate Decimal to Percent Calculator
Calculating the conversion from a decimal to a percent is straightforward. Here’s how you can do it step-by-step:
Step 1: Identify the Decimal
- Start by determining the decimal number that you want to convert into a percentage. This could be any number, not necessarily between 0 and 1.
Step 2: Multiply by 100
- Multiply the decimal by 100. This shifts the decimal point two places to the right, effectively converting the decimal into a percentage.
Step 3: Append the Percent Sign
- Add the percent sign (%) to the result from Step 2. This finalizes the conversion, indicating that the number is now a percentage.
Decimal to Percent Calculator Formula
The formula to convert a decimal to a percentage is:
Percentage=Decimal×100
Explanation of the Formula
- Decimal: This is the original number you have, which is not yet expressed in terms of parts per hundred. Decimals represent fractions, where the decimal point moves two places to the right to convert it into a percentage.
- Multiply by 100: Multiplying the decimal by 100 effectively shifts the decimal point two places to the right. This conversion aligns with the definition of a percentage, which is a number or ratio expressed as a fraction of 100.
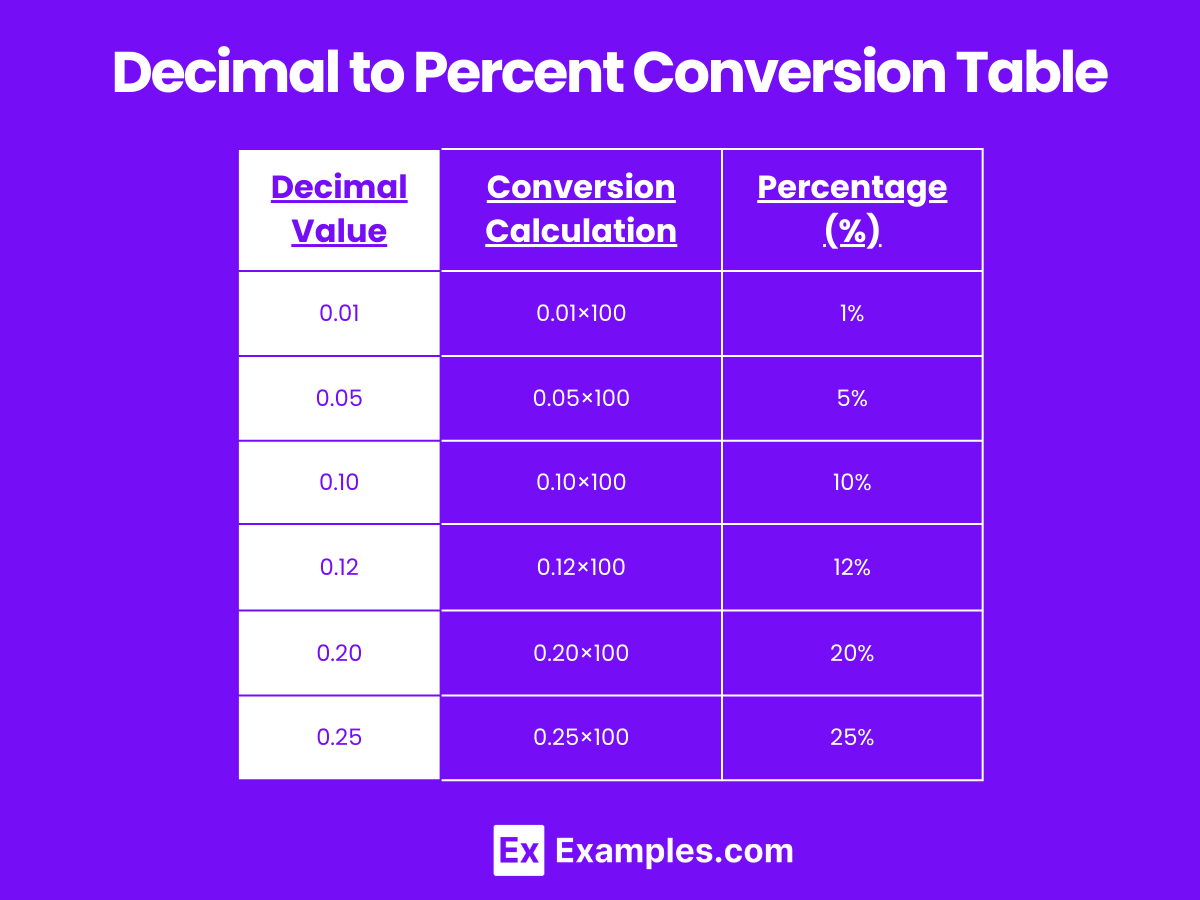
Decimal to Percent Conversion Table
| Decimal Value | Conversion Calculation | Percentage (%) |
|---|---|---|
| 0.01 | 0.01×100 | 1% |
| 0.05 | 0.05×100 | 5% |
| 0.10 | 0.10×100 | 10% |
| 0.12 | 0.12×100 | 12% |
| 0.20 | 0.20×100 | 20% |
| 0.25 | 0.25×100 | 25% |
| 0.33 | 0.33×100 | 33% |
| 0.50 | 0.50×100 | 50% |
| 0.75 | 0.75×100 | 75% |
| 0.80 | 0.80×100 | 80% |
| 0.90 | 0.90×100 | 90% |
| 1.00 | 1.00×100 | 100% |
| 1.25 | 1.25×100 | 125% |
| 1.50 | 1.50×100 | 150% |
| 2.00 | 2.00×100 | 200% |
| 3.50 | 3.50×100 | 350% |
| 4.75 | 4.75×100 | 475% |
| 5.00 | 5.00×100 | 500% |
| 10.00 | 10.00×100 | 1000% |
| 20.00 | 20.00×100 | 2000% |
Decimal to Percent Conversion Chart
Examples of Decimal to Percent Calculator
Example 1: Converting a Simple Decimal
- Decimal: 0.45
- Conversion: 0.45×100
- Result: 45%
Explanation: Multiplying 0.45 by 100 shifts the decimal point two places to the right, converting it to 45%.
Example 2: Converting a Small Decimal
- Decimal: 0.07
- Conversion: 0.07×100
- Result: 7%
Explanation: Multiplying 0.07 by 100 converts it to 7%, representing a small fraction in percentage form.
Example 3: Converting a Decimal Greater Than One
- Decimal: 1.23
- Conversion: 1.23×100
- Result: 123%
Explanation: When the decimal is greater than one, the percentage will be more than 100. Multiplying 1.23 by 100 gives 123%.
Example 4: Converting a Very Small Decimal
- Decimal: 0.003
- Conversion: 0.003×100
- Result: 0.3%
Explanation: Multiplying a very small decimal, such as 0.003, by 100 results in a percentage of 0.3%, which might represent a tiny fraction in statistical data.
Example 5: Converting a Decimal Involving Fractions
- Decimal: 0.625
- Conversion: 0.625×100
- Result: 62.5%
Explanation: Decimals that are exact fractions can be easily converted to percentages that might look familiar. Multiplying 0.625 by 100 gives 62.5%, which is the equivalent of the fraction 5885.
These examples show how to convert different types of decimal values into percentages, ranging from small decimals to decimals greater than one, using the simple multiplication-by-100 method.
How accurate is a Decimal to Percent Calculator?
The accuracy of the calculator typically depends on the number of decimal places it allows. Most calculators are designed to be very accurate and can handle inputs with multiple decimal places.
Can I convert a negative decimal to a percentage?
Yes, negative decimals can also be converted to percentages. The process is the same, and the resulting percentage will also be negative, indicating a decrease or loss when compared to a base of zero.
Is there a limit to the number of decimal places I can use?
Generally, there is no practical limit to the number of decimal places you can enter, although the calculator might round the percentage to a certain number of decimal places for display purposes.
What should I do if the calculator gives an error?
If the calculator gives an error, double-check the decimal input for any non-numeric characters or mistakes. Ensure only valid decimal numbers are entered.
Why do I need to convert decimals to percentages?
Converting decimals to percentages can make numbers easier to understand, especially in contexts like finance, statistics, or performance metrics, where comparisons relative to a hundred are common.
Are there any common mistakes to avoid when converting decimals to percentages?
A common mistake is forgetting to move the decimal point two places to the right. It’s crucial to remember this step to ensure accurate conversion.

