Learning Objectives
By studying the Introduction to Enthalpy of Reaction for the AP Chemistry exam, you should be able to understand and define enthalpy and enthalpy of reaction, distinguish between exothermic and endothermic reactions, calculate enthalpy changes using Hess’s Law and bond enthalpies, and interpret calorimetry data to determine heat exchange. You should also be able to apply these principles to real-world scenarios, understand the significance of standard enthalpy changes, and accurately solve related problems to predict the heat effects of chemical reactions.
Introduction
Enthalpy of reaction, often symbolized as ΔH, is a crucial concept in thermochemistry that quantifies the heat change during a chemical reaction at constant pressure. It reflects the difference in enthalpy between reactants and products, indicating whether a reaction is exothermic (releasing heat) or endothermic (absorbing heat). Understanding enthalpy of reaction is fundamental for predicting the energy requirements and feasibility of chemical processes, and it plays a vital role in fields ranging from industrial chemistry to biochemistry. By analyzing enthalpy changes, scientists and engineers can design more efficient and sustainable chemical reactions.
What is Enthalpy?
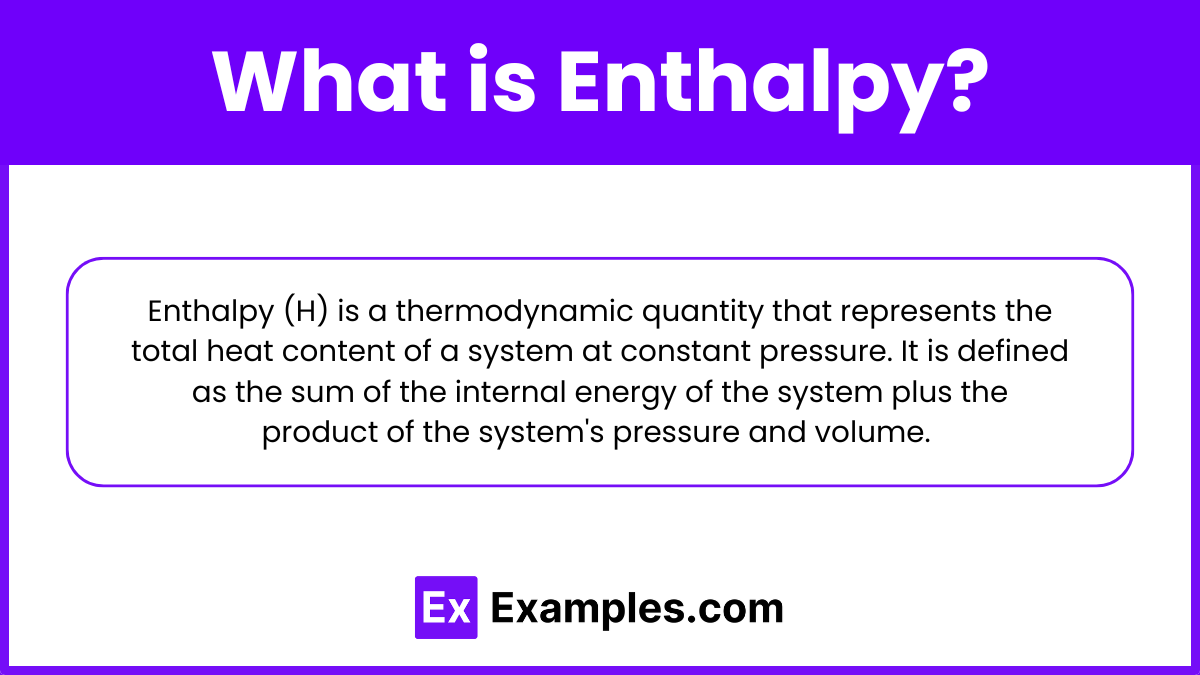
Enthalpy (H) is a thermodynamic quantity that represents the total heat content of a system at constant pressure. It is defined as the sum of the internal energy of the system plus the product of the system’s pressure and volume. Enthalpy is a state function, meaning its value depends only on the current state of the system and not on the path taken to reach that state. It is commonly measured in joules (J) or kilojoules (kJ).
Formula for Enthalpy
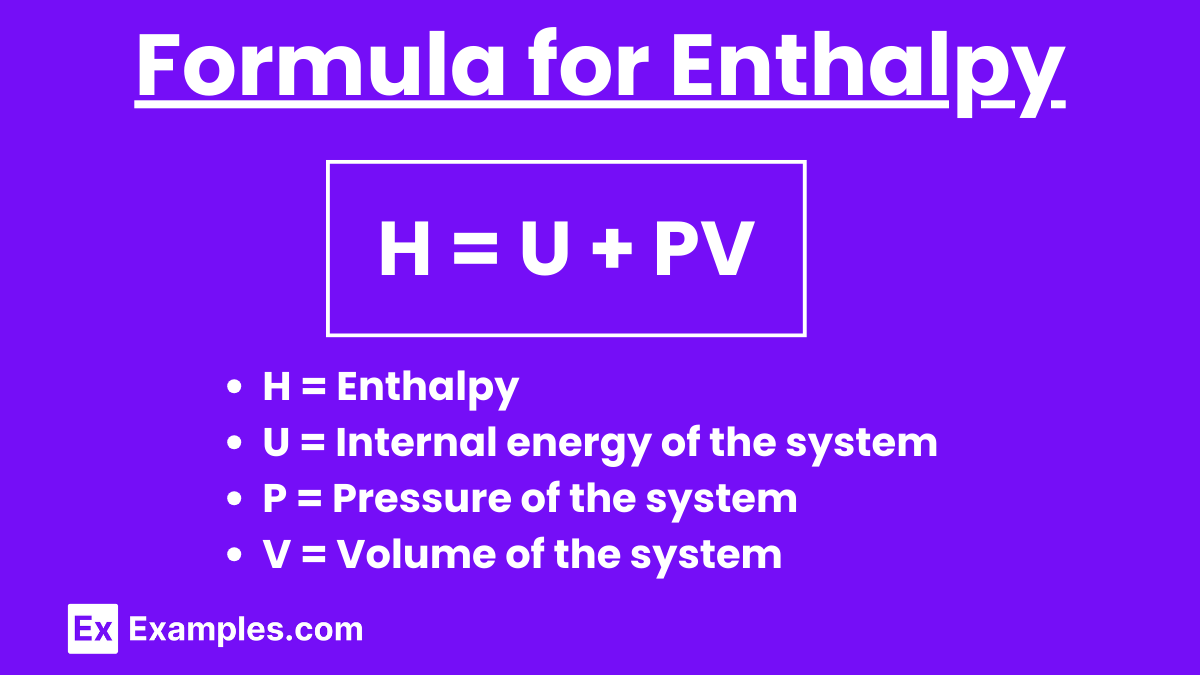
The equation defines enthalpy as the sum of the internal energy (U) and the product of the pressure (P) and volume (V) of the system.
H = U + PV
Where:
- H = Enthalpy
- U = Internal energy of the system
- P = Pressure of the system
- V = Volume of the system
Enthalpy of Reaction (ΔH)
The enthalpy of reaction (ΔH) is the change in enthalpy that occurs during a chemical reaction. It indicates whether a reaction releases or absorbs heat. This can be expressed as:
![]()
Types of Enthalpy Changes
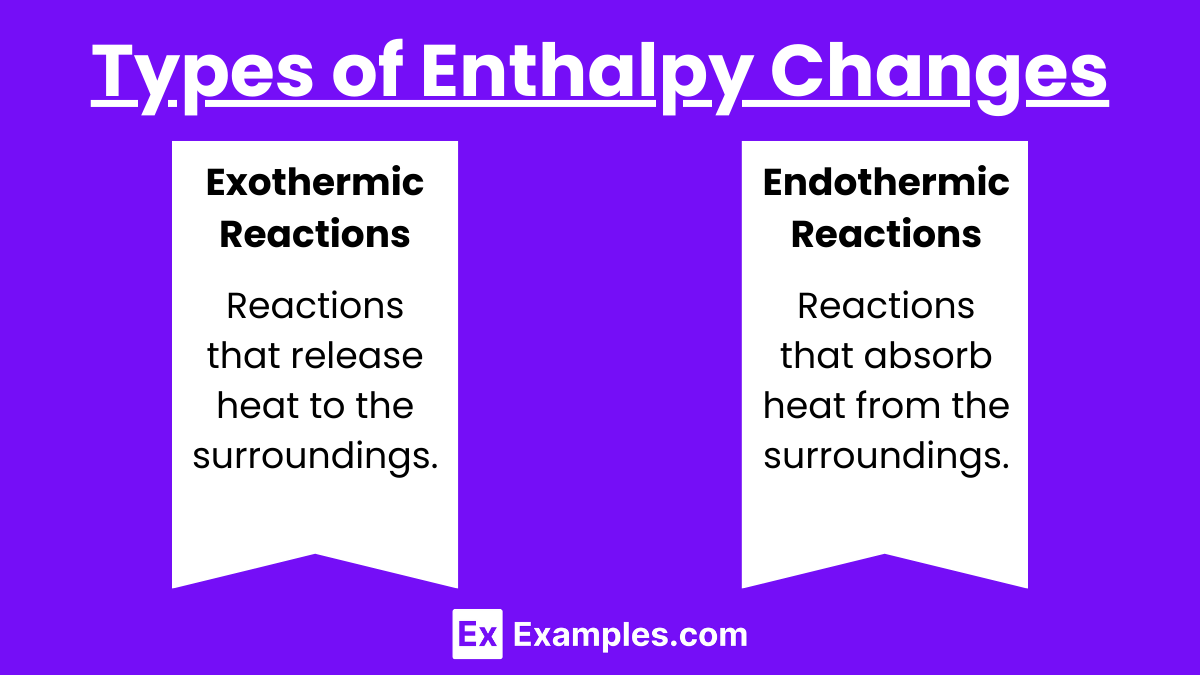
- Exothermic Reactions:
- Definition: Reactions that release heat to the surroundings.
- Sign of ΔH: Negative (ΔH < 0)
- Example: Combustion of methane. CH₄ + 2O₂ → CO₂ + 2H₂O ΔH=−890 kJ/mol
- Endothermic Reactions:
- Definition: Reactions that absorb heat from the surroundings.
- Sign of ΔH: Positive (ΔH > 0)
- Example: Decomposition of calcium carbonate. CaCO₃ → CaO + CO₂ ΔH = +178 kJ/mol
Standard Enthalpy of Reaction (ΔH°)
The standard enthalpy of reaction (ΔH°) is the enthalpy change that occurs when a reaction is carried out under standard conditions. Standard conditions typically mean a pressure of 1 atm and a temperature of 298 K (25°C).
Formula for Standard Enthalpy of Reaction
The standard enthalpy change of a reaction can be calculated using the standard enthalpies of formation (ΔH°բ) of the reactants and products. The standard enthalpy of formation is the enthalpy change when one mole of a compound is formed from its elements in their standard states.
![]()
Steps to Calculate ΔH°
- Identify the Products and Reactants: Write the balanced chemical equation for the reaction.
- Find Standard Enthalpies of Formation: Look up the standard enthalpies of formation (ΔH°բ) for all reactants and products involved in the reaction.
- Apply the Formula: Substitute the ΔH°բ values into the formula to calculate the standard enthalpy change of the reaction.
Hess’s Law
Hess’s Law states that the total enthalpy change for a chemical reaction is the same, regardless of the number of steps in the reaction pathway. This principle is based on the fact that enthalpy is a state function, meaning it depends only on the initial and final states of the system, not on the path taken.
Statement of Hess’s Law
The enthalpy change of an overall process is the sum of the enthalpy changes of its individual steps:
ΔHₜₒₜₐₗ = ΔH₁ + ΔH₂ + ΔH₃ +…
Steps to Use Hess’s Law
- Identify the Target Reaction: Write the balanced chemical equation for the reaction for which you want to determine ΔH.
- Break Down into Known Reactions: Find reactions with known enthalpy changes that can be combined to give the target reaction.
- Adjust Reactions: If necessary, reverse or multiply the reactions to match the stoichiometry of the target reaction. Remember to change the sign of ΔH when reversing a reaction and to multiply ΔH by the same factor when multiplying the reaction.
- Sum the Enthalpy Changes: Add the enthalpy changes of the individual steps to get the total enthalpy change for the target reaction.
Calculating Enthalpy Change
Calculating the enthalpy change (ΔH) of a reaction involves determining the heat absorbed or released during a chemical reaction. There are several methods to calculate ΔH, including using standard enthalpies of formation, bond enthalpies, and calorimetry data.
Method 1: Using Standard Enthalpies of Formation (ΔH°բ)
The standard enthalpy change of a reaction can be calculated using the standard enthalpies of formation of the reactants and products.
![]()
Steps:
- Write the balanced chemical equation.
- Look up the standard enthalpies of formation (ΔH°_f) for each reactant and product.
- Apply the formula to calculate ΔH° for the reaction.
Method 2: Using Bond Enthalpies
Bond enthalpy (also known as bond dissociation energy) is the energy required to break one mole of a bond in a gaseous molecule. The enthalpy change of a reaction can be estimated using bond enthalpies:
![]()
Steps:
- Write the balanced chemical equation.
- Identify all bonds broken and formed during the reaction.
- Look up the bond enthalpies for each bond.
- Apply the formula to calculate ΔH.
Method 3: Using Calorimetry
Calorimetry is an experimental method to measure the heat change of a reaction. This involves using a calorimeter to measure the temperature change of the surroundings.
q = mcΔT
Where:
- q= heat absorbed or released
- m = mass of the substance
- c = specific heat capacity
- ΔT = change in temperature
Steps:
- Measure the mass of the substance involved in the reaction.
- Measure the temperature change during the reaction.
- Use the specific heat capacity of the substance.
- Apply the formula to calculate the heat change (q).
- For reactions at constant pressure, ΔH ≈ q.