Learning Objectives
By studying Le Chatelier’s Principle for the AP Chemistry exam, you should be able to define and explain the principle, predict the direction of shift in equilibrium when a chemical system is disturbed by changes in concentration, pressure, or temperature, and understand the role of catalysts in equilibrium reactions. You should be able to apply this principle to various chemical reactions, analyze how equilibrium positions change in response to different stresses, and solve related equilibrium problems accurately. Mastery of these concepts will help you interpret and predict the behavior of chemical systems in dynamic equilibrium.
Free AP Chemistry Practice Test
Introduction
Le Chatelier’s Principle is a fundamental concept in chemistry that explains how a dynamic equilibrium system responds to changes in concentration, temperature, or pressure. This principle states that if an external change is imposed on a system at equilibrium, the system will adjust itself to partially counteract the effect of the change and restore a new equilibrium state. Understanding Le Chatelier's Principle helps predict the direction in which a reaction will shift when subjected to external stress, making it crucial for optimizing chemical processes in industrial applications and understanding natural phenomena.
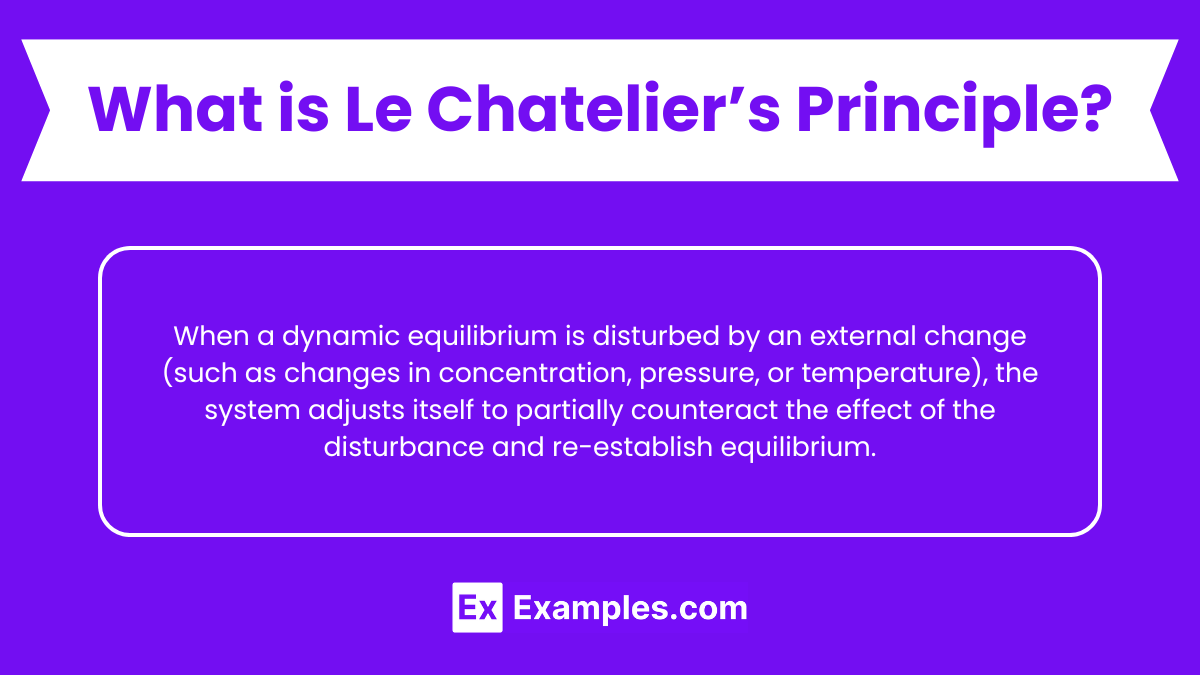
What is Le Chatelier’s Principle?
When a dynamic equilibrium is disturbed by an external change (such as changes in concentration, pressure, or temperature), the system adjusts itself to partially counteract the effect of the disturbance and re-establish equilibrium.
Dynamic Equilibrium
Dynamic equilibrium occurs in a chemical reaction when the rate of the forward reaction equals the rate of the reverse reaction. At this point, the concentrations of reactants and products remain constant over time, but they are not necessarily equal. This balance happens because the reactions are still occurring, but they are happening at the same rate in both directions, resulting in no net change in the concentration of reactants and products.
Key Characteristics of Dynamic Equilibrium
Constant Concentrations: The concentrations of reactants and products do not change over time.
Equal Reaction Rates: The rates of the forward and reverse reactions are equal.
Reversible Reactions: Dynamic equilibrium can only be established in reversible reactions.
Closed System: Equilibrium is maintained in a closed system where no substances are added or removed.
Example
Consider the reversible reaction:
At dynamic equilibrium, the rate at which N₂ and H₂ combine to form NH₃ is equal to the rate at which NH₃ decomposes back into N₂ and H₂. Although the reactions continue to occur, the overall concentrations of N₂, H₂ , and NH₃ remain constant.
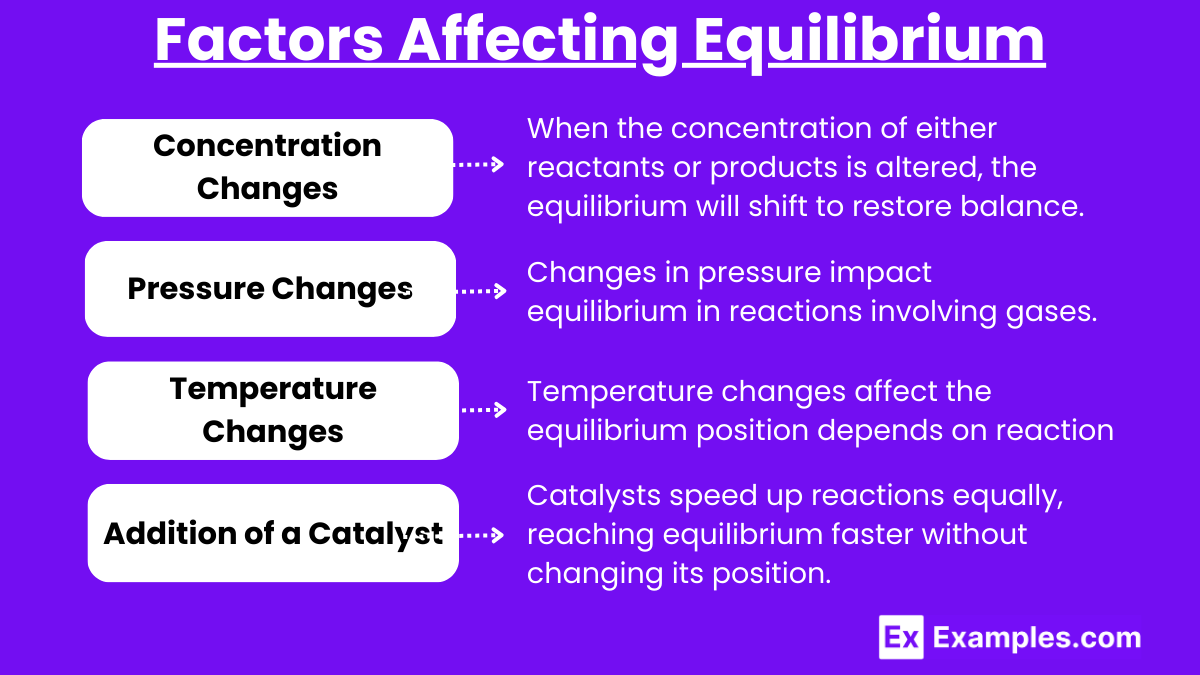
Factors Affecting Equilibrium
Several factors can disturb the equilibrium of a chemical system, prompting it to shift in a direction that counteracts the disturbance. These factors include changes in concentration, pressure, temperature, and the addition of a catalyst.
1. Concentration Changes
When the concentration of either reactants or products is altered, the equilibrium will shift to restore balance.
Increasing Reactant Concentration: Shifts the equilibrium to the right (toward the products) to use up the added reactants.
Decreasing Reactant Concentration: Shifts the equilibrium to the left (toward the reactants) to produce more of the depleted reactants.
Increasing Product Concentration: Shifts the equilibrium to the left (toward the reactants) to use up the added products.
Decreasing Product Concentration: Shifts the equilibrium to the right (toward the products) to produce more of the depleted products.
2. Pressure Changes (Affects Gaseous Systems)
Changes in pressure impact equilibrium in reactions involving gases. The system will shift to minimize the change in pressure.
Increasing Pressure: Shifts the equilibrium toward the side with fewer moles of gas, reducing the pressure.
Decreasing Pressure: Shifts the equilibrium toward the side with more moles of gas, increasing the pressure.
3. Temperature Changes
Temperature changes affect the equilibrium position depending on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
Exothermic Reactions:
Increasing Temperature: Shifts the equilibrium to the left (toward the reactants) as the system tries to absorb the added heat.
Decreasing Temperature: Shifts the equilibrium to the right (toward the products) as the system tries to produce more heat.
Endothermic Reactions:
Increasing Temperature: Shifts the equilibrium to the right (toward the products) as the system tries to absorb the added heat.
Decreasing Temperature: Shifts the equilibrium to the left (toward the reactants) as the system tries to produce more heat.
4. Addition of a Catalyst
Catalysts: Catalysts speed up the rate of both the forward and reverse reactions equally, allowing the system to reach equilibrium faster. However, they do not affect the position of the equilibrium.
Applications
Industrial Synthesis of Ammonia (Haber Process)
Adjusting pressure and temperature to maximize ammonia production.
Removing ammonia from the reaction mixture to drive the equilibrium toward product formation.
Production of Sulfuric Acid (Contact Process)
Optimizing the conditions to favor the production of sulfur trioxide, a key intermediate.
Using high pressures and low temperatures to shift the equilibrium toward product formation.
Carbonated Beverages
Maintaining high pressure to keep carbon dioxide dissolved in the liquid.
Release of pressure (opening the bottle) shifts the equilibrium, causing CO₂ to escape and produce bubbles.
Blood Oxygenation
Oxygen binding to hemoglobin is influenced by CO₂ levels, temperature, and pH.
High CO₂ concentrations or increased acidity shift equilibrium to release oxygen to tissues.
Environmental Chemistry
Managing emissions and pollutants by understanding their chemical equilibria.
Controlling conditions to minimize harmful chemical concentrations in the atmosphere and water bodies.
Summary Table
Change Factor | Direction of Equilibrium Shift | Example |
|---|---|---|
Increase [Reactant] | Right (toward products) | Adding N₂ in the Haber Process |
Decrease [Reactant] | Left (toward reactants) | Removing H₂ in the Haber Process |
Increase [Product] | Left (toward reactants) | Adding NH₃ in the Haber Process |
Decrease [Product] | Right (toward products) | Removing NH₃ in the Haber Process |
Increase Pressure | Toward fewer gas moles | Increasing pressure in the Haber Process |
Decrease Pressure | Toward more gas moles | Decreasing pressure in the Haber Process |
Increase Temperature (Exothermic) | Left (toward reactants) | Heating the exothermic Contact Process |
Decrease Temperature (Exothermic) | Right (toward products) | Cooling the exothermic Contact Process |
Increase Temperature (Endothermic) | Right (toward products) | Heating an endothermic reaction |
Decrease Temperature (Endothermic) | Left (toward reactants) | Cooling an endothermic reaction |
Add Catalyst | No shift, equilibrium reached faster | Catalyst in any reversible reaction |
Examples of Le Chatelier’s Principle
1. Haber Process for Ammonia Synthesis
Reaction:
N₂(g) + 3H₂(g) ↔ 2NH₃(g) + heat
Increasing Pressure: Shifts right, producing more NH₃.
Decreasing Temperature: Shifts right, favoring the exothermic reaction.
2. Contact Process for Sulfuric Acid Production
Reaction:
2SO₂(g) + O₂(g) ↔ 2SO₃(g)+heat
Increasing Pressure: Shifts right, producing more SO₃.
Decreasing Temperature: Shifts right, favoring the exothermic reaction.
3. Carbonated Beverages
Reaction:
CO₂(g) ↔ CO₂(aq)
Increasing Pressure: Shifts right, more CO₂ dissolves.
Decreasing Pressure (Opening a Bottle): Shifts left, CO₂ escapes as gas.
4. Blood Oxygenation
Reaction:
Hb(aq) + O₂(g) ↔ HbO₂(aq)
High CO₂ (Tissues): Shifts left, oxygen is released.
Low CO₂ (Lungs): Shifts right, oxygen is absorbed.
5. Buffer Solutions
Reaction:
CH₃COOH(aq) ↔ CH₃COO⁻(aq) + H⁺ (aq)
Adding Acid (Increasing (H⁺): Shifts left, reducing H⁺ concentration.
Adding Base (Decreasing (H⁺): Shifts right, increasing H⁺ concentration.