Learning Objectives
By mastering Lewis diagrams for the AP Chemistry exam, you should be able to accurately depict the valence electron arrangements of atoms and molecules, predict molecular geometry, determine the type and strength of chemical bonds, identify and draw resonance structures, and calculate formal charges to assess the stability of different electron configurations. These skills will enable you to understand and predict the chemical behavior and reactivity of various substances.
Introduction
Lewis diagrams is also known as Lewis dot structures, are a visual representation of the valence electrons in atoms and molecules. Developed by Gilbert N. Lewis, these diagrams illustrate how atoms bond together by sharing or transferring electrons to achieve stable electron configurations, typically an octet. By representing the distribution of electrons around atoms, Lewis diagrams helps to predict the arrangement, bonding, and reactivity of molecules.
What is Lewis Diagrams?
Lewis diagrams is also known as Lewis dot structures, are graphical representations that depict the valence electrons of atoms within a molecule. These diagrams show how the electrons are arranged around individual atoms and how they participate in chemical bonding, illustrating both shared (bonding) and lone (non-bonding) pairs of electrons.
Basic Principles of Lewis Diagrams
- Valence Electrons:
- Valence electrons are the outermost electrons of an atom.
- They determine an atom’s chemical properties and its ability to bond with other atoms.
- Lewis Symbols:
- Represent an atom and its valence electrons.
- The element’s symbol is surrounded by dots representing valence electrons.
- Example: The Lewis symbol for oxygen (O) has 6 dots around the symbol O.
Importance of Lewis Diagrams
- Predicting Molecular Shape: Helps determine the geometry of molecules using the VSEPR theory.
- Understanding Bonding: Illustrates how atoms share electrons to form covalent bonds and achieve stable configurations.
- Determining Formal Charges: Assists in identifying the most stable electron arrangement in molecules.
- Visualizing Electron Distribution: Shows the distribution of valence electrons among atoms in a molecule.
- Identifying Lone Pairs: Highlights lone pairs of electrons that can affect molecular shape and reactivity.
- Explaining Reactivity: Provides insights into how molecules interact and react based on their electron configurations.
Steps to Draw Lewis Diagrams
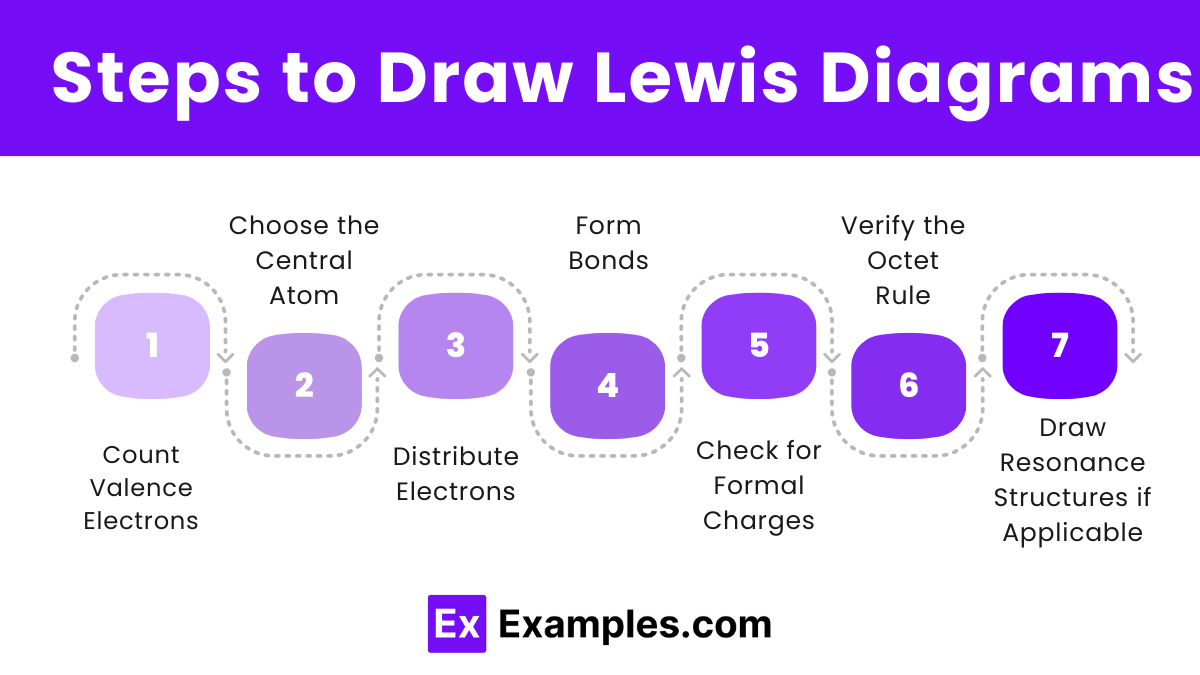
1. Determine the Total Number of Valence Electrons
Count the valence electrons for all atoms in the molecule. For neutral molecules, sum the valence electrons of each atom. For ions, add an electron for each negative charge or subtract one for each positive charge.
Example: For water (H₂O), hydrogen has 1 valence electron and oxygen has 6, so the total is 2×1+6=8 Valence electrons.
2. Choose the Central Atom
The central atom is typically the least electronegative element, except for hydrogen, which is never the central atom. The central atom is the one that can form the most bonds.
Example: In carbon dioxide (CO₂), carbon is the central atom because it is less electronegative than oxygen and can form four bonds.
3. Distribute Electrons Around the Atoms
Place a pair of electrons (a single bond) between the central atom and each surrounding atom to represent bonding pairs. Then distribute the remaining electrons as lone pairs to satisfy the octet rule for each atom, starting with the outer atoms and moving to the central atom.
Example: For methane (CH₄), carbon forms four single bonds with four hydrogen atoms, using all 8 valence electrons.
4. Form Double or Triple Bonds if Necessary
If there are not enough electrons to complete the octet for each atom, form double or triple bonds by sharing more pairs of electrons between atoms. Ensure that the total number of electrons remains the same.
Example: In carbon dioxide (CO₂), carbon forms two double bonds with two oxygen atoms to complete the octets, using all 16 valence electrons.
5. Check for Formal Charges
Calculate the formal charge for each atom to verify the most stable structure. The formal charge is given by the formula: Formal Charge = Valence Electrons – Lone Pair Electrons + ![]()
The most stable Lewis structure has the smallest formal charges, with negative formal charges preferably on the more electronegative atoms.
Example: For sulfate ion (SO₄²⁻), sulfur and oxygen atoms may share electrons in different ways, but the structure with the formal charges closest to zero and any negative charges on oxygen (the more electronegative atom) is the most stable.
6. Verify the Octet Rule
Ensure that each atom (except hydrogen) has an octet of electrons. If any atom does not, reconsider the placement of electrons or the formation of multiple bonds.
Example: For ammonia (NH₃), nitrogen forms three single bonds with hydrogen and has one lone pair, satisfying the octet rule for nitrogen.
7. Draw Resonance Structures if Applicable
If a molecule can be represented by multiple valid Lewis structures, draw all possible resonance structures. These structures illustrate different ways to arrange the electrons that are equivalent and contribute to the overall hybrid structure of the molecule.
Example: For ozone (O₃), there are two resonance structures where the double bond and single bond switch places between the oxygen atoms.
Examples of Lewis Diagrams
Methane (CH₄)
- Valence Electrons: 4 (C) + 4 × 1 (H) = 8
- Central Atom: Carbon
- Single Bonds: C forms four single bonds with H
- Lewis Structure:
H – C – H
2. Water (H₂O)
- Valence Electrons: 6 (O) + 2 × 1 (H) = 8
- Central Atom: Oxygen
- Single Bonds: O forms two single bonds with H
- Lewis Structure:
H – O – H
3. Carbon Dioxide (CO₂)
- Valence Electrons: 4 (C) + 2 × 6 (O) = 16
- Central Atom: Carbon
- Double Bonds: C forms two double bonds with O
- Lewis Structure:
O = C = O
4. Ammonia (NH₃)
- Valence Electrons: 5 (N) + 3 × 1 (H) = 8
- Central Atom: Nitrogen
- Single Bonds: N forms three single bonds with H
- Lone Pair: One lone pair on N
- Lewis Structure:
H – N – H
Resonance Structures
Resonance structures are different Lewis diagrams that represent the same molecule. They show different possible arrangements of electrons while keeping the positions of the atoms fixed. These structures help describe delocalized electrons within certain molecules or polyatomic ions where a single Lewis diagram cannot accurately depict the true electronic structure.
Characteristics of Resonance Structures
- Same Arrangement of Atoms: Atoms are placed in the same positions in all resonance structures.
- Different Electron Arrangements: Only the positions of electrons (particularly lone pairs and pi bonds) differ among the structures.
- Equivalence in Energy: Resonance structures usually have similar energies, contributing equally to the resonance hybrid.
- Resonance Hybrid: The actual structure of the molecule is a hybrid of all resonance structures, representing a more accurate depiction of electron distribution.
Importance of Resonance Structures
- Stabilization: Resonance structures can explain the stabilization of molecules due to delocalization of electrons.
- Distribution of Charges: Helps to understand the distribution of charges in a molecule.
- Reactivity and Properties: Provides insight into the reactivity and other properties of molecules.
Examples of Resonance Structures
1. Ozone (O₃)
- Valence Electrons: 18 (6 for each O)
- Lewis Structures:
a) O – O = O b) O = O – O
Each oxygen-oxygen bond is a mix of single and double bonds, making the bonds equivalent in the resonance hybrid.
Exceptions to the Octet Rule
1. Incomplete Octets
Some atoms are stable with fewer than eight electrons in their valence shell. These typically include small atoms where achieving an octet is not possible.
2. Expanded Octets
Atoms in the third period and beyond can have more than eight electrons in their valence shell. This is because they have access to the d-orbitals, which can accommodate additional electrons.
3. Odd-Electron Molecules
Some molecules have an odd number of valence electrons, resulting in at least one atom having an unpaired electron. These molecules, often referred to as free radicals, are typically very reactive.
4. Molecules with Formal Charges
Some stable molecules have atoms that do not have a full octet, typically due to the presence of formal charges. These formal charges arise when the distribution of electrons does not allow for an octet around each atom.
Practice Problems
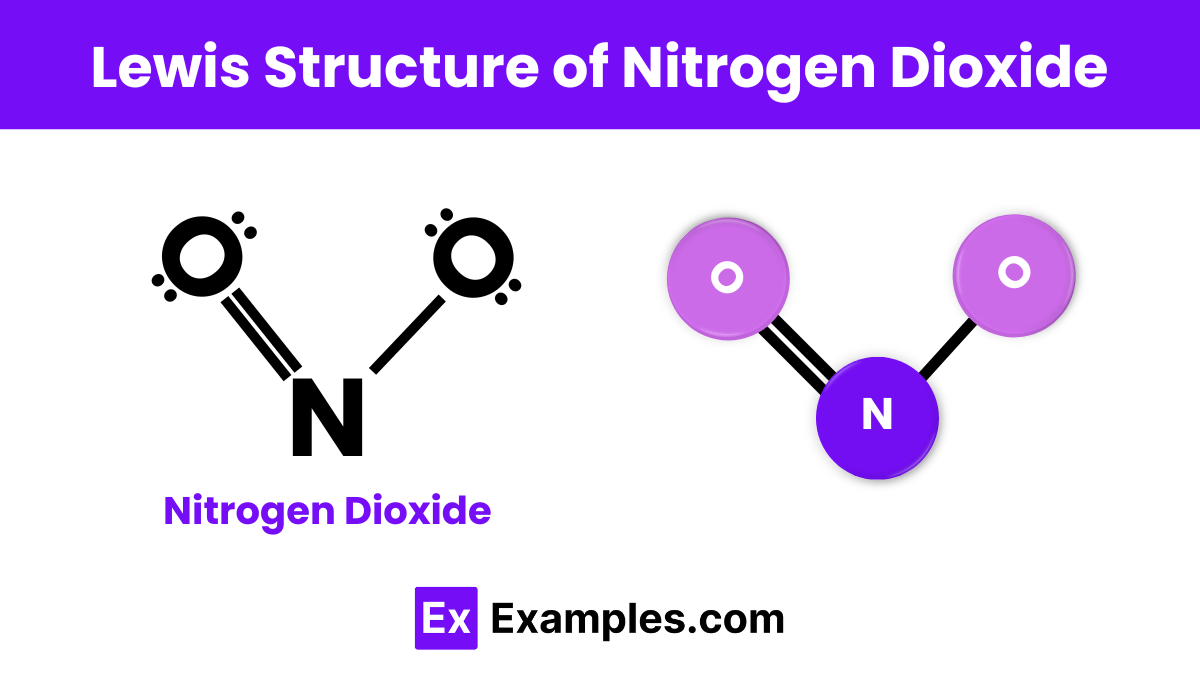
Draw the Lewis structure for nitrogen dioxide (NO₂).
Nitrogen dioxide (NO₂) has an odd number of valence electrons (17), making it an example of an odd-electron molecule.
- Count Valence Electrons:
- Nitrogen (N): 5 valence electrons
- Oxygen (O): 6 valence electrons × 2 = 12 electrons
- Total: 5 + 12 = 17 valence electrons
- Draw Skeleton Structure:
- O – N – O
- Distribute Remaining Electrons:
- Place lone pairs on the oxygen atoms to complete their octets.
- Place remaining electrons on nitrogen.
- Due to an odd number of electrons, one electron will be unpaired.
- Lewis Diagram:
Find the formal charges in the Lewis structure of phosphate ion (PO₄³⁻).
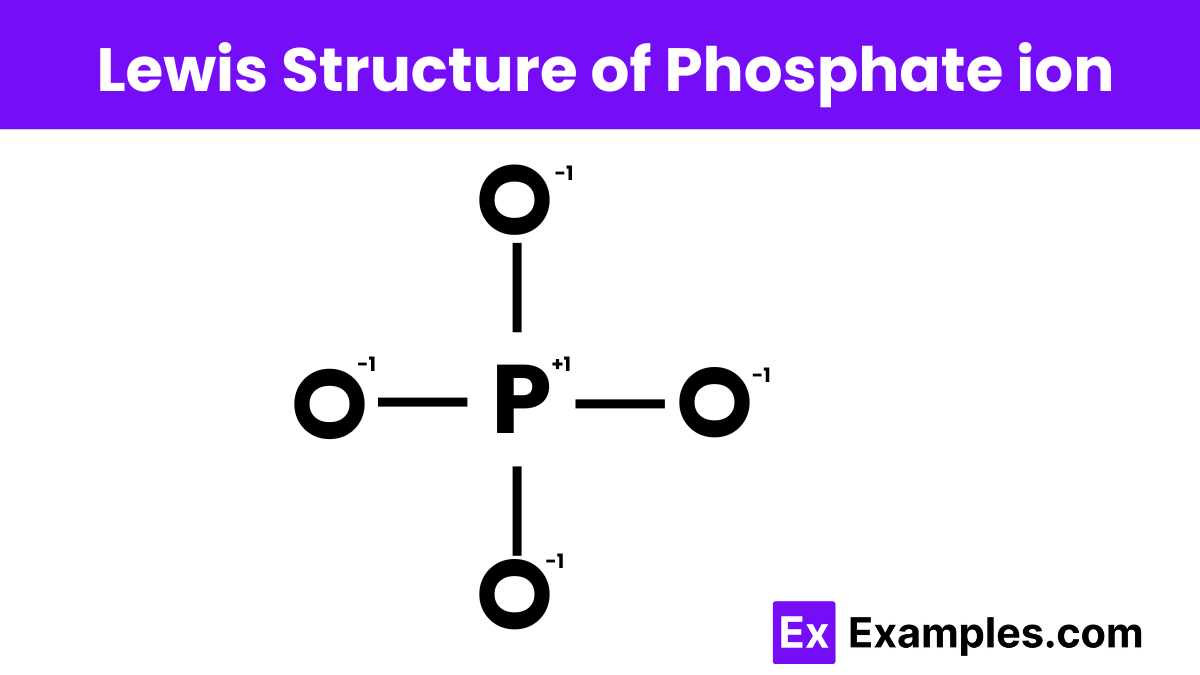
Phosphate ion (PO₄³⁻) has a total of 32 valence electrons.
- Determine the Total Number of Valence Electrons:
- Phosphorus (P) has 5 valence electrons.
- Oxygen (O) has 6 valence electrons.
- The extra three negative charges add 3 more electrons.
- Total = 5 + 4 × 6 + 3 = 32 valence electrons.
- Distribute the Electrons:
- Place single bonds between P and each O.
- Distribute remaining electrons to complete the octets around O atoms.
- Calculate Formal Charges:
- Formal Charge (FC) = Valence Electrons – (Lone Pair Electrons + 1/2 Bonding Electrons)
- Phosphorus (P): 5 – (0 + 4) = +1
- Each Oxygen (O): 6 – (6 + 1) = -1
Thus, the formal charges for the phosphate ion are +1 for phosphorus and -1 for each oxygen atom.