Learning Objectives
In studying the molecular structure of acids and bases for the AP Chemistry exam, you should aim to understand the definitions and classifications of acids and bases according to the Bronsted-Lowry and Lewis theories. You should be able to identify and describe the molecular structures of various acids and bases, including binary acids, oxyacids, carboxylic acids, metal hydroxides, amine bases, and oxyanion bases. You should learn how electronegativity, bond strength, resonance, and inductive effects influence acid and base strength. Additionally, you should be able to recognize conjugate acid-base pairs, predict the outcomes of acid-base reactions, and compare the relative strengths of different acids and bases based on their molecular structures. Understanding these concepts will enable you to analyze and solve related problems effectively.
Introduction
The molecular structure of acids and bases is key to their properties and behavior. Acids donate protons (H⁺ ions), often having hydrogen atoms bonded to electronegative elements. Bases accept protons and usually contain lone pairs of electrons or negatively charged ions. Strong acids and bases fully dissociate in water, while weak ones only partially dissociate. This structural understanding helps predict their reactivity and applications in various fields.
Acids
What are Acids?

An acid is a substance that can donate a proton (H⁺) to another substance or, according to the Lewis theory, accept an electron pair. In aqueous solutions, acids increase the concentration of hydrogen ions (H⁺). Common characteristics of acids include a sour taste, the ability to turn blue litmus paper red, and the capacity to react with bases to form water and salts.
Types of Acids
1. Binary Acids
Binary acids consist of hydrogen and one other non-metal element. They have the general formula HX, where H is hydrogen and X is the non-metal.
- Examples: Hydrochloric acid (HCl), Hydrobromic acid (HBr), Hydrofluoric acid (HF).
- Properties: The strength of binary acids depends on the bond strength between hydrogen and the non-metal. Generally, as the bond strength decreases and the electronegativity of X increases, the acid strength increases.
2. Oxyacids
Oxyacids contain hydrogen, oxygen, and another element (usually a non-metal). They have the general formula HnEOm, where E is the central element.
- Examples: Sulfuric acid (H₂SO₄), Nitric acid (HNO₃), Phosphoric acid (H₃PO₄).
- Properties: The acid strength of oxyacids depends on the electronegativity of the central atom and the number of oxygen atoms bonded to it. More electronegative central atoms and higher numbers of oxygen atoms generally increase acid strength due to increased electron withdrawal from the hydrogen atom.
3. Carboxylic Acids
Carboxylic acids are organic acids that contain the carboxyl group (-COOH).
- Examples: Acetic acid (CH₃COOH), Formic acid (HCOOH), Benzoic acid (C₆H₅COOH).
- Properties: The carboxyl group can release a proton (H⁺), and the resulting carboxylate ion (R-COO⁻) is stabilized by resonance. The acid strength is influenced by the nature of the R group; electron-withdrawing groups increase acid strength by stabilizing the conjugate base, while electron-donating groups decrease acid strength.
4. Strong Acids
Strong acids completely ionize in aqueous solutions, meaning they donate protons very readily.
- Examples: Hydrochloric acid (HCl), Sulfuric acid (H₂SO₄), Nitric acid (HNO₃).
- Properties: Strong acids have high acid dissociation constants (Ka) and low pKa values, indicating complete or near-complete dissociation in water.
5. Weak Acids
Weak acids partially ionize in aqueous solutions, meaning they donate protons less readily.
- Examples: Acetic acid (CH₃COOH), Formic acid (HCOOH), Citric acid (C₆H₈O₇).
- Properties: Weak acids have lower acid dissociation constants (Ka) and higher pKa values, indicating incomplete dissociation in water.
6. Polyprotic Acids
Polyprotic acids can donate more than one proton per molecule. They have multiple dissociation steps, each with its own Ka.
- Examples: Sulfuric acid (H₂SO₄), Phosphoric acid (H₃PO₄), Carbonic acid (H₂CO₃).
- Properties: The first dissociation step usually has a higher Ka value (stronger acid) than subsequent steps. Each subsequent proton is more difficult to remove, making the acid progressively weaker.
7. Lewis Acids
Lewis acids are defined by their ability to accept an electron pair.
- Examples: Aluminum chloride (AlCl₃), Boron trifluoride (BF₃), Iron(III) chloride (FeCl₃).
- Properties: Lewis acids do not need to contain hydrogen. They are often metal cations or molecules with electron-deficient atoms that can accept electron pairs from Lewis bases.
Molecular Structure of Acids
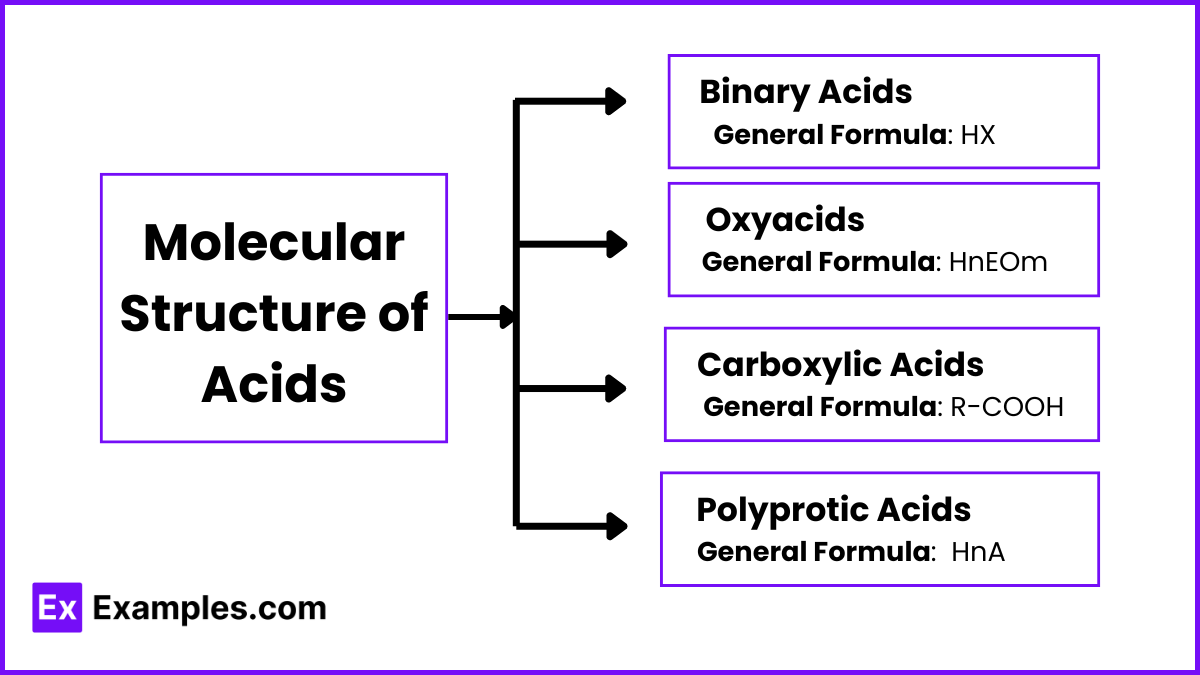
Understanding the molecular structure of acids is crucial in predicting their behavior, strength, and reactivity. Different types of acids have unique structural characteristics that influence their ability to donate protons (H⁺).
1. Binary Acids
General Formula: HX (where X is a non-metal)
- Example: Hydrochloric Acid (HCl)
- Structure: A single bond between hydrogen (H) and a halogen (X).
- Properties:
- Bond Strength: The H-X bond strength decreases down the group in the periodic table, making acids like HCl, HBr, and HI stronger as X becomes larger and less electronegative.
- Electronegativity: Higher electronegativity of X increases the polarity of the H-X bond, facilitating proton donation.
2. Oxyacids
General Formula: HnEOm (where E is a central atom, usually a non-metal, and O is oxygen)
- Example: Sulfuric Acid (H₂SO₄)
- Structure: One or more hydrogen atoms bonded to oxygen atoms, which are bonded to a central atom (E).
- Properties:
- Electronegativity of E: A higher electronegativity of the central atom increases the acid strength.
- Number of Oxygen Atoms: More oxygen atoms increase acid strength by stabilizing the negative charge on the conjugate base through resonance.
- Resonance: The ability of the conjugate base to delocalize its negative charge through resonance structures enhances acid strength.
3. Carboxylic Acids
General Formula: R-COOH (where R is an organic group)
- Example: Acetic Acid (CH₃COOH)
- Structure: Contains a carboxyl group (-COOH) where a carbon atom is double-bonded to an oxygen atom (carbonyl group) and single-bonded to a hydroxyl group (-OH).
- Properties:
- Resonance Stabilization: The conjugate base (R-COO⁻) is stabilized by resonance, spreading the negative charge over two oxygen atoms, making it easier for the molecule to donate a proton.
- Inductive Effect: Electron-withdrawing groups attached to the carbon chain increase acid strength by stabilizing the conjugate base. Electron-donating groups decrease acid strength.
4. Polyprotic Acids
General Formula: HnA (where A is the central atom and n is the number of ionizable hydrogen atoms)
- Example: Phosphoric Acid (H₃PO₄)
- Structure: Contains multiple hydrogen atoms that can be sequentially donated, each step having its own dissociation constant (Ka).
- Properties:
- Sequential Dissociation: The first proton is usually the easiest to lose, and subsequent protons are harder to remove due to increasing negative charge on the anion.
- Ka Values: The first dissociation constant (Ka1) is higher than the second (Ka2), and so on.
5. Lewis Acids
Definition: Acids that accept an electron pair (do not necessarily contain hydrogen).
- Example: Aluminum Chloride (AlCl₃)
- Structure: Often involves a central atom with empty orbitals that can accept electron pairs from a Lewis base.
- Properties:
- Electron Deficiency: The central atom has an incomplete octet and is capable of accepting electron pairs.
- Coordination Compounds: Lewis acids can form coordination compounds by accepting electron pairs from ligands.
Acid Strength and Structure
The strength of an acid depends on its ability to donate protons (H⁺). This ability is influenced by the molecular structure of the acid. Here, we will explore the factors that affect acid strength and how different structural elements contribute to this property.
Factors Affecting Acid Strength
- Bond Strength: Weaker H-X bonds increase acid strength (e.g., HI > HBr > HCl > HF).
- Electronegativity: Higher electronegativity of the central atom increases acid strength (e.g., HClO₄ > HClO).
- Resonance Stabilization: Stabilization of the conjugate base increases acid strength (e.g., acetic acid > ethanol).
- Inductive Effect: Electron-withdrawing groups increase acid strength, while electron-donating groups decrease it (e.g., CF₃COOH > CH₃COOH).
- Hybridization: Greater s-character in hybridized orbitals increases acid strength (e.g., C₂H₂ (sp) > C₂H₄ (sp²) > C₂H₆ (sp³)).
Bases
What are Bases?
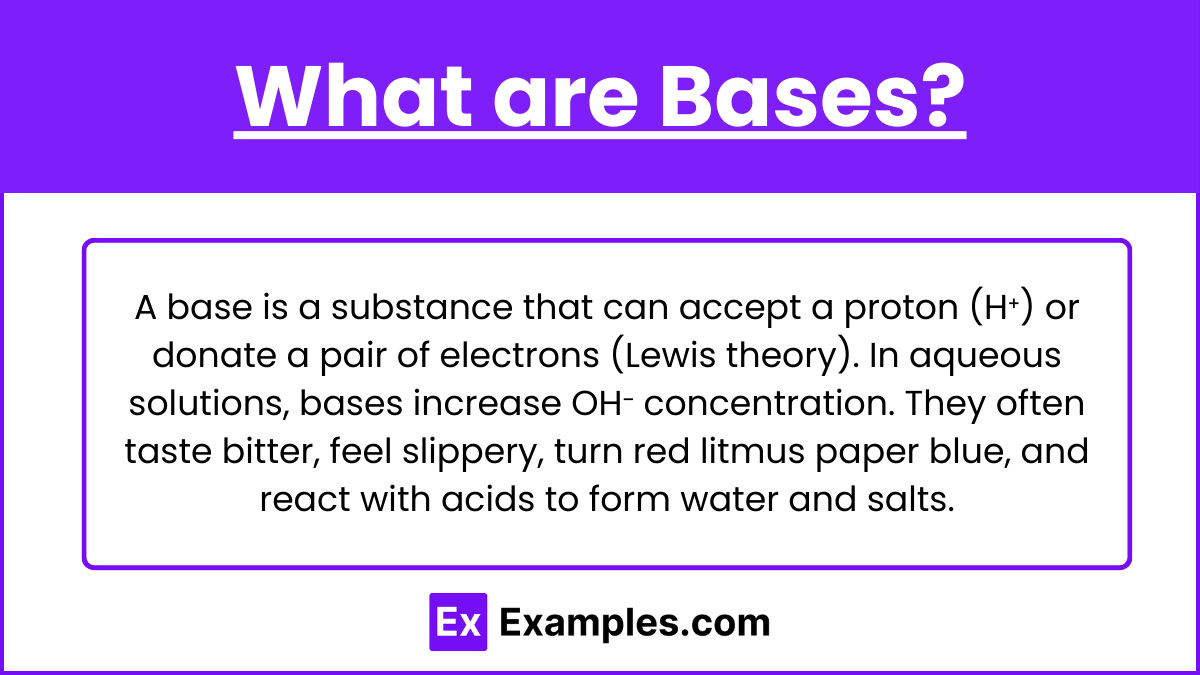
A base is a substance that can accept a proton (H⁺) or, according to the Lewis theory, donate a pair of electrons. In aqueous solutions, bases increase the concentration of hydroxide ions (OH⁻). Bases often have a bitter taste, feel slippery to the touch, turn red litmus paper blue, and react with acids to form water and salts.
Types of Bases
1. Metal Hydroxides
Definition: Compounds that contain metal ions bonded to hydroxide ions (OH⁻).
- Examples: Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Calcium hydroxide (Ca(OH)₂).
- Properties:
- Strong bases.
- Fully dissociate in water to release OH⁻ ions.
- Commonly used in various industrial and chemical processes.
2. Amine Bases
Definition: Organic compounds containing nitrogen atoms with a lone pair of electrons that can accept protons.
- Examples: Ammonia (NH₃), Methylamine (CH₃NH₂), Ethylamine (C₂H₅NH₂).
- Properties:
- Weak to moderate bases.
- Partially ionize in water.
- Nitrogen’s lone pair is responsible for accepting protons, making them bases.
3. Oxyanion Bases
Definition: Anions derived from oxyacids, capable of accepting protons.
- Examples: Nitrate (NO₃⁻), Sulfate (SO₄²⁻), Phosphate (PO₄³⁻).
- Properties:
- Strength varies depending on the conjugate acid.
- Often found as components of salts in water.
4. Strong Bases
Definition: Bases that completely dissociate in water to release OH⁻ ions.
- Examples: Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Barium hydroxide (Ba(OH)₂).
- Properties:
- High base dissociation constant (Kb).
- Fully ionize in solution.
- Very effective in increasing the OH⁻ concentration in aqueous solutions.
5. Weak Bases
Definition: Bases that partially dissociate in water.
- Examples: Ammonia (NH₃), Pyridine (C₅H₅N), Acetate ion (CH₃COO⁻).
- Properties:
- Lower base dissociation constant (Kb).
- Only partially ionize in solution.
- Less effective in increasing the OH⁻ concentration compared to strong bases.
6. Lewis Bases
Definition: Compounds that donate an electron pair.
- Examples: Ammonia (NH₃), Ethanol (C₂H₅OH), Ethylene (C₂H₄).
- Properties:
- Not limited to substances containing OH⁻.
- Often contain lone pairs of electrons that can be donated to a Lewis acid.
Molecular Structure of Bases
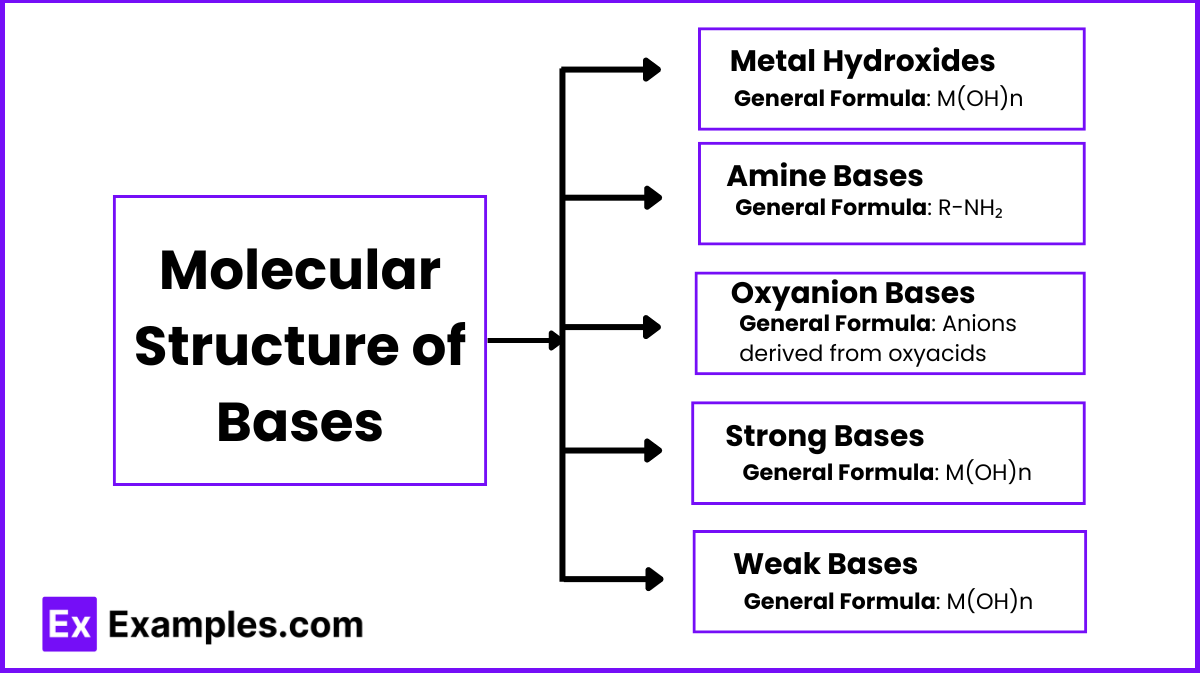
The molecular structure of bases plays a significant role in their ability to accept protons (H⁺) or donate electron pairs. Here, we will explore the structural characteristics of various types of bases and how these structures influence their basicity.
1. Metal Hydroxides
General Formula: M(OH)n (where M is a metal and n is the charge of the metal ion)
- Example: Sodium Hydroxide (NaOH)
- Structure: Composed of metal cations (Na⁺) and hydroxide anions (OH⁻).
- Properties:
- In aqueous solution, metal hydroxides dissociate completely to release OH⁻ ions.
- Strong bases due to full dissociation in water.
2. Amine Bases
General Formula: R-NH₂, R₂NH, or R₃N (where R is an alkyl or aryl group)
- Example: Ammonia (NH₃)
- Structure: Nitrogen atom with a lone pair of electrons that can accept a proton.
- Properties:
- The lone pair on nitrogen is responsible for the base’s ability to accept protons.
- Weak to moderate bases, as they only partially ionize in water.
- Basicity can be influenced by the nature of the R groups; electron-donating groups increase basicity.
3. Oxyanion Bases
General Formula: Anions derived from oxyacids (e.g., NO₃⁻, SO₄²⁻)
- Example: Nitrate (NO₃⁻)
- Structure: Central atom (often a non-metal) bonded to oxygen atoms, with one or more oxygen atoms carrying a negative charge.
- Properties:
- The negative charge on the oxygen atoms makes these anions capable of accepting protons.
- The basicity is influenced by the stability of the conjugate acid and the distribution of the negative charge.
4. Strong Bases
General Formula: Typically metal hydroxides like M(OH)n
- Example: Potassium Hydroxide (KOH)
- Structure: Similar to metal hydroxides, consisting of metal cations and hydroxide anions.
- Properties:
- Completely dissociate in water to release OH⁻ ions.
- Very effective in increasing the concentration of OH⁻ in solution.
5. Weak Bases
General Formula: Varies widely (e.g., R-NH₂, anions like CH₃COO⁻)
- Example: Ammonia (NH₃)
- Structure: May include lone pairs on nitrogen atoms or negative charges on anions.
- Properties:
- Only partially dissociate or ionize in water.
- The basicity is influenced by the availability of lone pairs or the stability of the conjugate acid.
6. Lewis Bases
Definition: Compounds that donate an electron pair
- Example: Ammonia (NH₃)
- Structure: Contain atoms (usually nitrogen or oxygen) with lone pairs of electrons.
- Properties:
- Can form coordinate covalent bonds by donating an electron pair to a Lewis acid.
- Not limited to substances that contain hydroxide ions.
Base Strength and Structure
The strength of a base depends on its ability to accept protons (H⁺) or donate electron pairs. Several structural factors influence this ability, including the availability of lone pairs, electronegativity, resonance stabilization, inductive effects, and hybridization.
Factors Affecting Base Strength
- Lone Pair Availability: Readily available lone pairs increase base strength (e.g., NH₃).
- Electronegativity: Lower electronegativity increases base strength (e.g., amines > alcohols).
- Resonance Stabilization: Resonance decreases base strength (e.g., CH₃COO⁻ < CH₃CH₂O⁻).
- Inductive Effect: Electron-withdrawing groups decrease base strength; electron-donating groups increase it (e.g., CF₃CH₂NH₂ < CH₃CH₂NH₂).
- Hybridization: Greater s-character decreases base strength (e.g., C₂H₂ < C₂H₄).