Understanding moles and molar mass is fundamental to mastering AP Chemistry. This topic is the cornerstone of stoichiometry, chemical reactions, and quantitative chemistry.
Free AP Chemistry Practice Test
Learning Objectives:
By the end of this lesson, students should be able to define a mole and understand it as a unit of measurement using Avogadro's number (6.022 x 10²³). They should be able to calculate molar mass and convert between mass, moles, and number of particles. Students will learn to define and calculate molarity, perform dilution calculations, and relate moles to chemical equations and stoichiometry, including identifying the limiting reactant and calculating theoretical yield. They should also be able to apply the ideal gas law and perform gas stoichiometry calculations. Finally, students will relate the concept of moles to real-world contexts, including laboratory experiments and practical applications in various fields. Key terms include mole, Avogadro's number, molar mass, molarity, stoichiometry, limiting reactant, and ideal gas law.
The Mole Concept
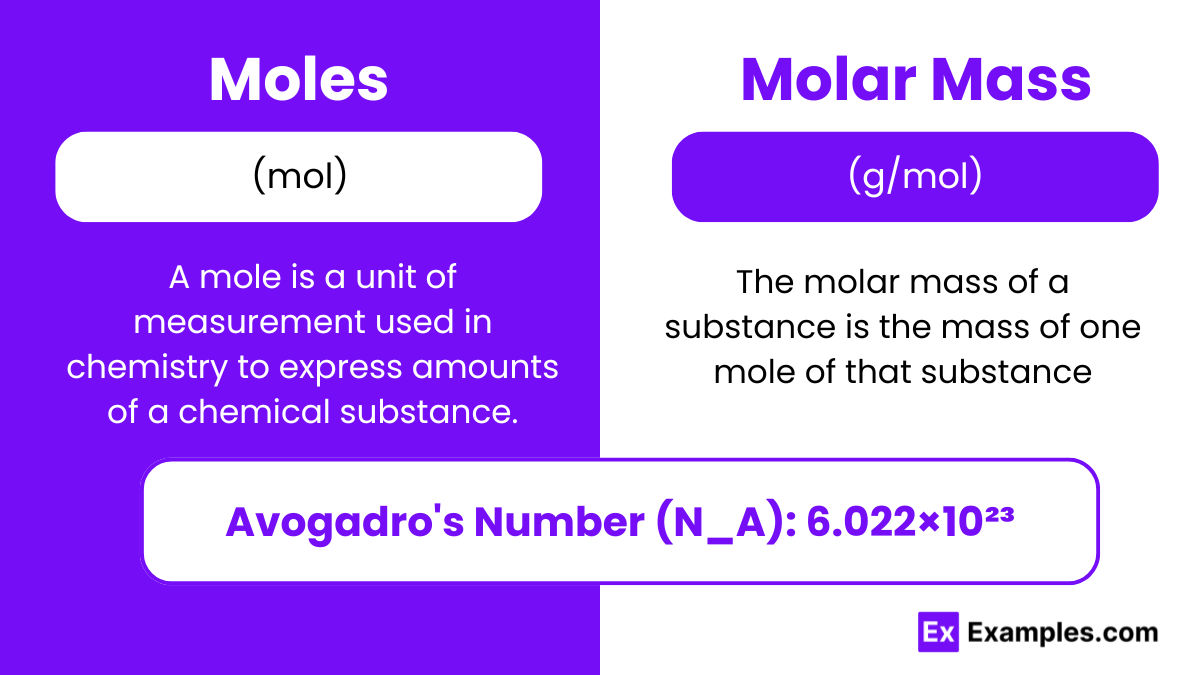
What is Mole? - Definition
A mole is a unit of measurement used in chemistry to express amounts of a chemical substance. The mole (symbol: mol) is defined as the amount of any substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are in 12 grams of pure carbon-12 (¹²C). This number is known as Avogadro's number, which is approximately 6.022×1023.
Avogadro's Number
Avogadro's Number (N_A): 6.022×1023
Represents the number of atoms, molecules, or particles in one mole of a substance.
Essential for converting between atomic mass units (amu) and grams.
Importance of the Mole
Provides a bridge between the atomic world (atoms and molecules) and the macroscopic world (grams and liters).
Allows chemists to count particles by weighing them.
Molar Mass
What is Molar Mass? - Definition
The molar mass of a substance is the mass of one mole of that substance. It is expressed in grams per mole (g/mol). The molar mass of an element is numerically equal to its atomic mass in unified atomic mass units (u or amu).
How to Calculate Molar Mss
Here are the simple steps to calculate molar mass
Identify the Elements
Determine which elements are in the compound and their quantities.
Find Atomic Mass:
Look up the atomic masses of each element on the periodic table.
Multiply and Sum
Multiply the atomic mass of each element by the number of atoms of that element in the molecule, then sum these values.
Example: Calculating the molar mass of H₂O
Hydrogen (H): Atomic mass = 1.008 amu
Oxygen (O): Atomic mass = 16.00 amu
Step 1: Molar mass of H₂O=(2×1.008 g/mol)+(1×16.00 g/mol)
Step 2: Molar mass of H₂O=2.016 g/mol+16.00 g/mol
Step 3: Molar mass of H₂O=18.016 g/mol
Common Molar Masses
Here are some common substances and their molar masses for quick reference:
NaCl (Sodium Chloride): 58.44 g/mol
H₂SO₄ (Sulfuric Acid): 98.08 g/mol
C₆H₁₂O₆ (Glucose): 180.16 g/mol
CO₂ (Carbon Dioxide): 44.01 g/mol
NH₃ (Ammonia): 17.03 g/mol
H₂O (Water): 18.016 g/mol
O₂ (Oxygen Gas): 32.00 g/mol
CH₄ (Methane): 16.04 g/mol
C₂H₆ (Ethane): 30.07 g/mol
C₃H₈ (Propane): 44.09 g/mol
C₄H₁₀ (Butane): 58.12 g/mol
C₂H₅OH (Ethanol): 46.07 g/mol
HCl (Hydrochloric Acid): 36.46 g/mol
NaOH (Sodium Hydroxide): 40.00 g/mol
KOH (Potassium Hydroxide): 56.11 g/mol
Ca(OH)₂ (Calcium Hydroxide): 74.10 g/mol
Mg(OH)₂ (Magnesium Hydroxide): 58.32 g/mol
NaHCO₃ (Sodium Bicarbonate): 84.01 g/mol
CaCO₃ (Calcium Carbonate): 100.09 g/mol
Na₂CO₃ (Sodium Carbonate): 105.99 g/mol
H₂O₂ (Hydrogen Peroxide): 34.01 g/mol
C₆H₆ (Benzene): 78.11 g/mol
C₈H₁₀ (Toluene): 92.14 g/mol
CH₃COOH (Acetic Acid): 60.05 g/mol
NH₄Cl (Ammonium Chloride): 53.49 g/mol
Fe₂O₃ (Iron(III) Oxide): 159.69 g/mol
FeSO₄ (Iron(II) Sulfate): 151.91 g/mol
CuSO₄ (Copper(II) Sulfate): 159.61 g/mol
Al₂O₃ (Aluminum Oxide): 101.96 g/mol
Pb(NO₃)₂ (Lead(II) Nitrate): 331.21 g/mol
AgNO₃ (Silver Nitrate): 169.87 g/mol
K₂SO₄ (Potassium Sulfate): 174.26 g/mol
KCl (Potassium Chloride): 74.55 g/mol
ZnSO₄ (Zinc Sulfate): 161.47 g/mol
CaCl₂ (Calcium Chloride): 110.98 g/mol
Using Moles and Molar Mass in Calculations
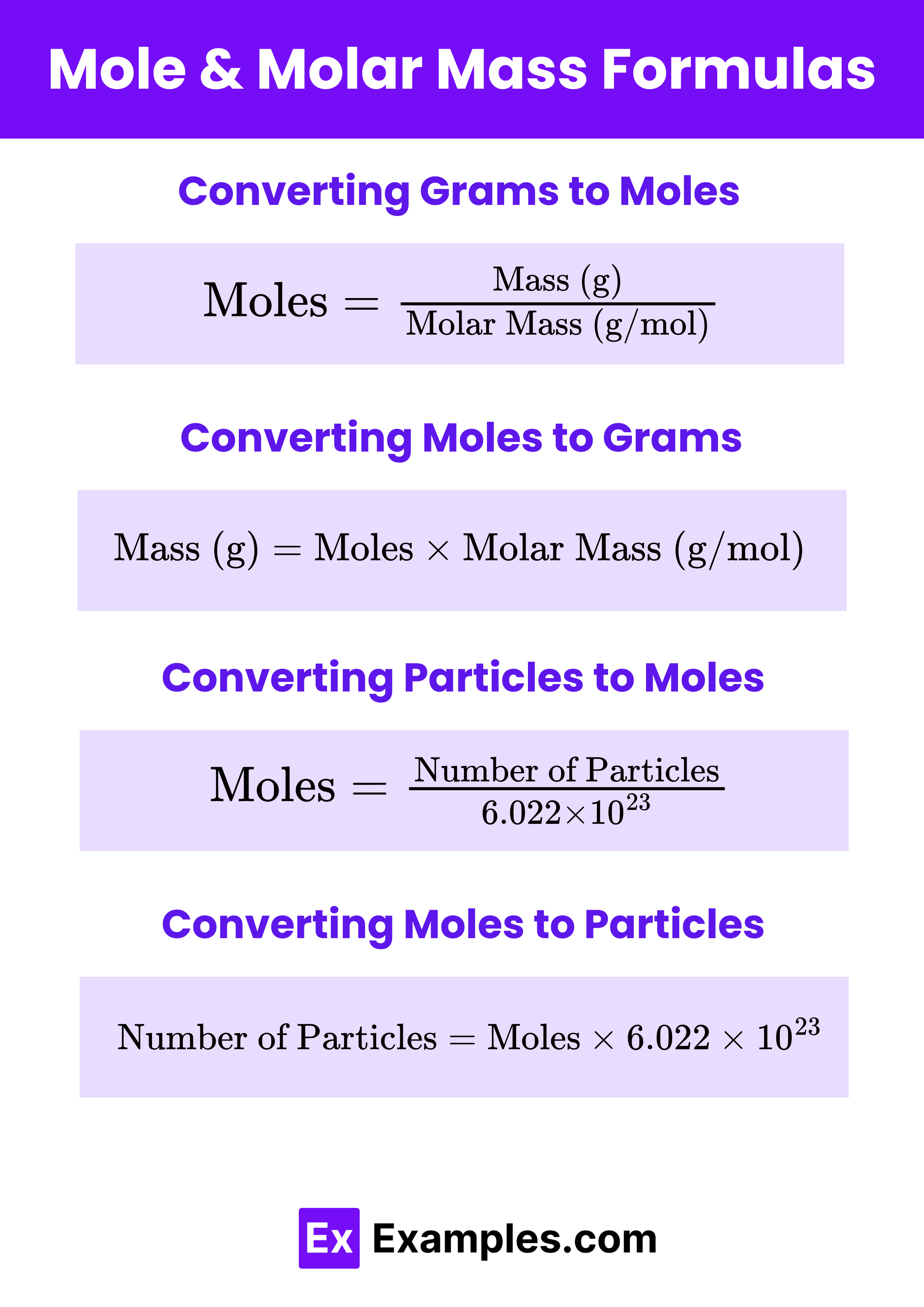
Download Moles & Molar Mass Formulas PDF
Converting Grams to Moles
To convert grams of a substance to moles, use the formula:
Example: Converting 36 grams of water (H₂O) to moles
Converting Moles to Grams
To convert moles of a substance to grams, use the formula:
Example: Converting 0.5 moles of water (H₂O) to grams
Mass of H₂O = 0.5 moles × 18.016 g/mol
Mass of H₂O ≈ 9.008 g
Converting Particles to Moles
To convert the number of particles (atoms, molecules, ions) to moles, use Avogadro's number:
Example: Converting 1.204×1024 molecules of CO₂ to moles
Converting Moles to Particles
To convert moles to the number of particles, multiply by Avogadro's number:
Number of Particles = Moles × 6.022×1023
Example: Converting 3 moles of H₂O to molecules
Number of Molecules of H₂O = 3 moles × 6.022 × 1023
Number of Molecules of H₂O = 1.807 × 1024 molecules
$ \text{Number of Molecules} = 1.807 \times 10^{24} \text{ molecules} $