Learning Objectives
In studying net ionic equations for the AP Chemistry exam, you should aim to master the following: identify and write balanced molecular equations for various chemical reactions, split strong electrolytes into their constituent ions, recognize and exclude spectator ions, and accurately write the net ionic equations that reflect only the species undergoing chemical change. Additionally, you should understand solubility rules to predict precipitate formation, be able to balance mass and charge in net ionic equations, and differentiate between different types of reactions such as precipitation, acid-base neutralization, and redox reactions. Mastery of these objectives will enhance your problem-solving skills and deepen your understanding of the fundamental chemical processes.
Introduction
Net ionic equations are a streamlined representation of chemical reactions that highlight the species directly involved in the reaction, excluding spectator ions that do not participate. These equations are essential for understanding the core chemical changes occurring in a reaction, as they simplify complex reactions into their most fundamental components. By focusing on the ions and molecules that undergo transformation, net ionic equations provide a clearer insight into the reactivity and interactions of substances in solution.
What are Net Ionic Equations?
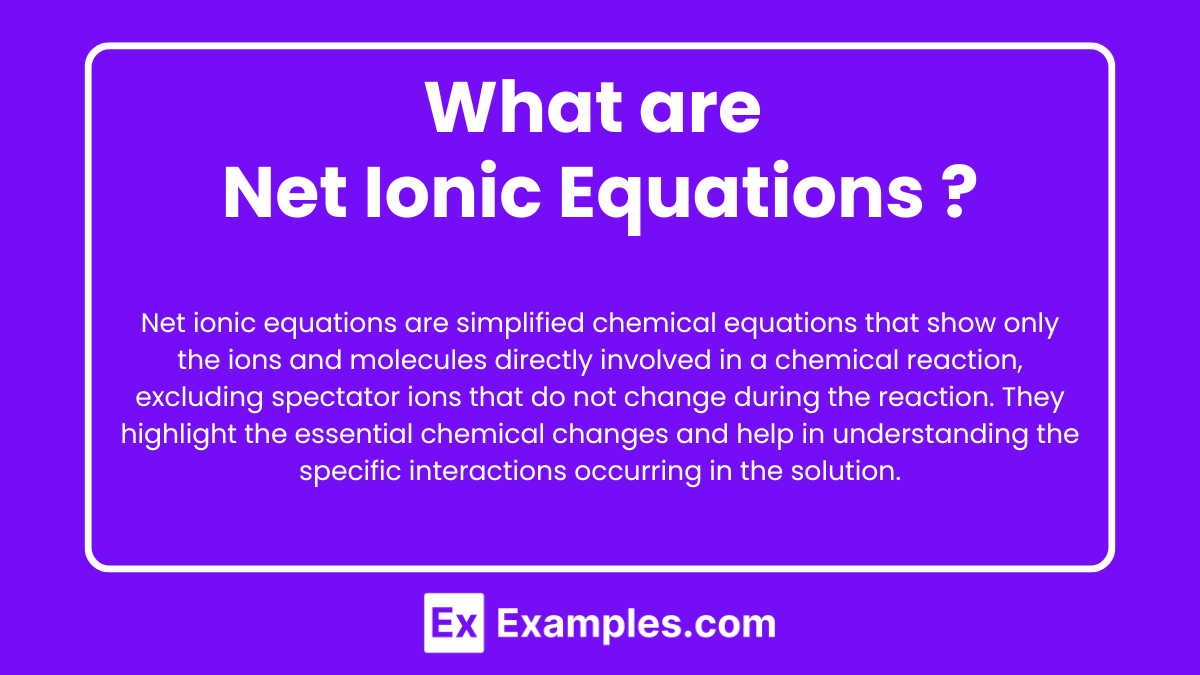
Net ionic equations are simplified chemical equations that show only the ions and molecules directly involved in a chemical reaction, excluding spectator ions that do not change during the reaction. They highlight the essential chemical changes and help in understanding the specific interactions occurring in the solution.
Types of Reactions and Their Net Ionic Equations
1. Precipitation Reactions
Description: Precipitation reactions occur when two aqueous solutions combine to form an insoluble solid, known as a precipitate. This type of reaction often involves the exchange of ions between the reacting solutions.
Example:
Net Ionic Equation:
2. Acid-Base Neutralization Reactions
Description: Acid-base neutralization reactions involve the reaction between an acid and a base to produce water and a salt. These reactions typically involve the transfer of protons (H⁺ ions) from the acid to the base.
Example:
Net Ionic Equation:
3. Redox (Oxidation-Reduction) Reactions
Description: Redox reactions involve the transfer of electrons between species, resulting in changes in oxidation states. One species undergoes oxidation (loss of electrons) while another undergoes reduction (gain of electrons).
Example:
Net Ionic Equation:
4. Double Displacement Reactions
Description: Double displacement reactions is also known as metathesis reactions, involve the exchange of ions between two compounds to form new compounds. These reactions often result in the formation of a precipitate, gas, or water.
Example:
Net Ionic Equation:
5. Single Displacement Reactions
Description: Single displacement reactions occur when one element displaces another in a compound. This type of reaction often involves a more reactive element displacing a less reactive element from a compound.
Example:
Net Ionic Equation:
6. Combustion Reactions
Description: Combustion reactions involve the reaction of a substance with oxygen to produce oxides and release energy in the form of heat and light. These reactions are typically exothermic and result in the formation of carbon dioxide and water when organic compounds combust.
Example:
Net Ionic Equation: Combustion reactions are usually written as molecular equations because they do not occur in aqueous solutions.
Steps to Write Net Ionic Equations

- Write the Balanced Molecular Equation
- Identify all reactants and products and ensure the equation is balanced in terms of atoms and charges.
- Split Strong Electrolytes into Ions
- Dissociate all strong electrolytes (soluble salts, strong acids, and strong bases) into their respective ions.
- Identify and Remove Spectator Ions
- Spectator ions are ions that appear unchanged on both sides of the equation. Eliminate them to focus on the actual chemical change.
- Write the Net Ionic Equation
- Include only the species that undergo a chemical change, omitting the spectator ions.
- Ensure Mass and Charge Balance
- Double-check that both the mass and the charge are balanced in the net ionic equation.
- Mass balance: 1 Ag atom and 1 Cl atom on both sides.
- Charge balance: +1 (Ag⁺) + (-1) (Cl⁻) = 0 on both sides.
How to Identify Net Ionic Equation
- Write the Complete Molecular Equation – Start with the full balanced equation of the reaction, including all reactants and products.
- Dissociate All Strong Electrolytes – Break down strong acids, strong bases, and soluble salts into their respective ions.
- Identify Spectator Ions – Locate ions that appear on both sides of the equation without undergoing any change.
- Eliminate Spectator Ions – Remove the spectator ions from the equation, as they do not participate in the actual reaction.
- Write the Net Ionic Equation – Include only the ions and molecules that participate in the chemical change.
- Check for Balance – Ensure the net ionic equation is balanced in terms of both mass and charge.
Uses of Net Ionic Equation
- Simplify Complex Reactions – Highlight the essential chemical changes without the distraction of spectator ions.
- Focus on Reactivity – Emphasize the actual species that undergo chemical change, providing clearer insight into reaction mechanisms.
- Predict Reaction Outcomes – Help predict the formation of products such as precipitates, gases, or water.
- Identify Reaction Types – Clarify whether a reaction is a precipitation, acid-base, or redox reaction.
- Aid in Stoichiometric Calculations – Assist in calculating the amounts of reactants and products involved in the reaction.
Common Mistakes to Avoid
- Incorrectly Identifying Spectator Ions – Ensure you accurately identify and remove all spectator ions.
- Not Balancing the Molecular Equation – Always balance the complete molecular equation before proceeding to the net ionic equation.
- Forgetting to Dissociate Strong Electrolytes – Remember to split all strong acids, bases, and soluble salts into ions.
- Omitting Phases – Always include the physical states (solid, liquid, gas, aqueous) of reactants and products.
- Imbalance in Charge or Mass – Double-check that both mass and charge are balanced in the final net ionic equation.
Practice Examples
Example 1:
- Molecular Equation:
- Net Ionic Equation:
Example 2:
- Molecular Equation:
- Net Ionic Equation:
Example 3:
- Molecular Equation:
- Net Ionic Equation: