Learning Objectives
By studying the pH and pOH of strong acids and bases for the AP Chemistry exam, you should be able to: understand the concept of complete dissociation in strong acids and bases; accurately calculate the hydrogen ion concentration [H⁺] and hydroxide ion concentration [OH⁻]; use these concentrations to determine the pH and pOH of solutions; apply the relationship pH + pOH = 14 to solve for unknowns; and confidently perform these calculations with common strong acids like HCl and HNO₃ and strong bases like NaOH and KOH, enhancing your ability to solve related problems on the exam.
Introduction
Understanding the pH and pOH of strong acids and bases is essential in chemistry. Strong acids and bases completely dissociate in water, resulting in high concentrations of hydrogen ions H⁺ or hydroxide ions OH⁻. The pH measures the acidity of a solution, while pOH measures its basicity. The sum of pH and pOH always equals 14.
What is pH of Strong Acids and Bases?
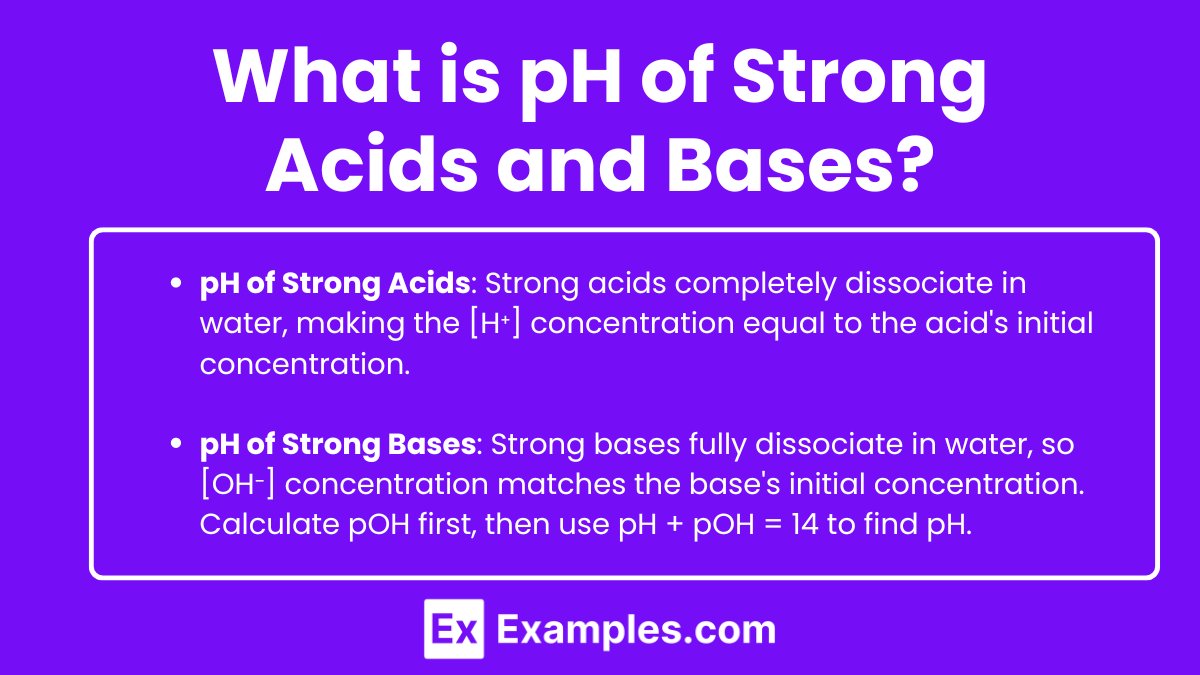
pH of Strong Acids
Strong acids fully dissociate in water, releasing all their hydrogen ions into the solution. The concentration of hydrogen ions [H⁺] in the solution directly equals the initial concentration of the acid.
pH of Strong Bases
Strong bases fully dissociate in water, releasing all their hydroxide ions into the solution. The concentration of hydroxide ions [OH⁻] in the solution directly equals the initial concentration of the base. To find the pH, first calculate the pOH and then use the relationship pH + pOH = 14.
What is pOH of Strong Acids and Bases?
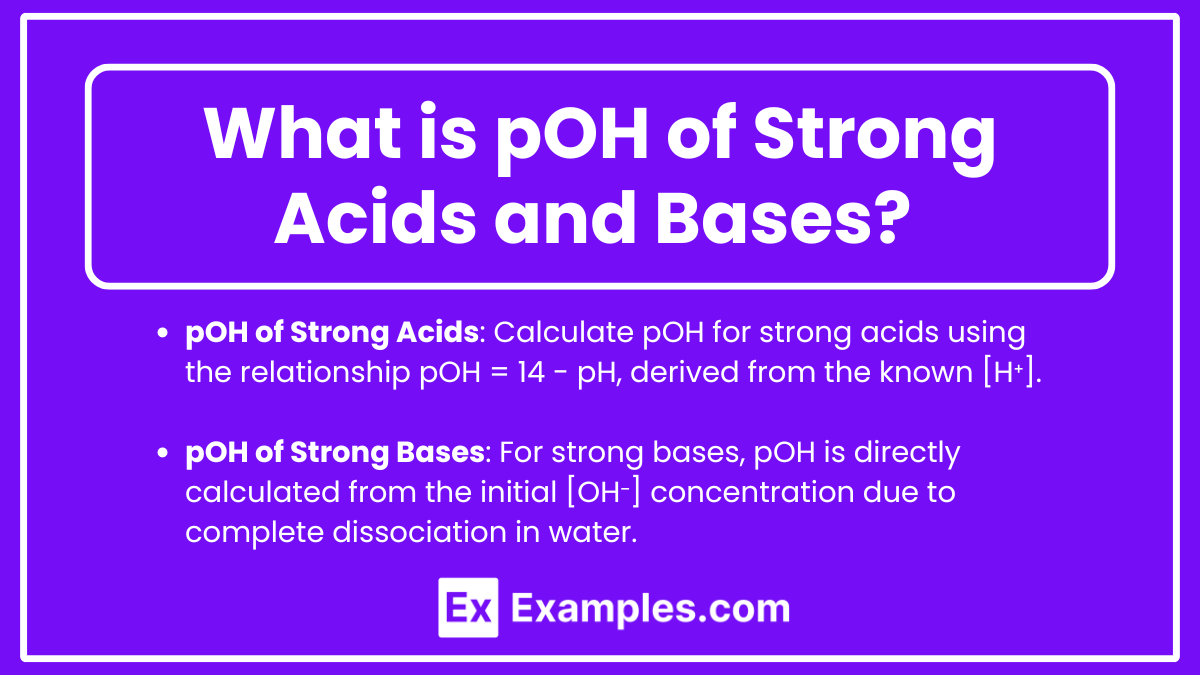
pOH of Strong Acids
While the pOH is not typically the primary focus when dealing with strong acids, it can still be calculated using the relationship between pH and pOH. For strong acids, the concentration of hydrogen ions [H⁺] is known, allowing for the determination of pOH.
pOH of Strong Bases
Strong bases fully dissociate in water, releasing all their hydroxide ions into the solution. The concentration of hydroxide ions [OH⁻] in the solution equals the initial concentration of the base. The pOH is calculated directly from [OH⁻].
Strong Acids
Strong acids are acids that completely dissociate in water, releasing all of their hydrogen ions (H⁺) into the solution. This complete ionization makes them very effective at increasing the hydrogen ion concentration in a solution, leading to a low pH. Here are some common examples of strong acids:
- Hydrochloric Acid (HCl)
- Found in stomach acid and used in industrial cleaning.
- Sulfuric Acid (H₂SO₄)
- Used in car batteries and industrial manufacturing processes.
- Nitric Acid (HNO₃)
- Commonly used in fertilizers and explosives.
- Perchloric Acid (HClO₄)
- Used in the production of rocket fuel and explosives.
- Hydrobromic Acid (HBr)
- Utilized in the synthesis of bromine compounds.
- Hydroiodic Acid (HI)
- Used in organic and inorganic iodine compound synthesis.
Strong Bases
Strong bases completely dissociate in water, releasing hydroxide ions [OH−][OH^-][OH−] into the solution. Here are five common strong bases along with their chemical formulas and typical uses:
- Sodium Hydroxide (NaOH)
- Manufacturing of soap and detergents
- Paper production
- Drain cleaners
- Potassium Hydroxide (KOH)
- Production of biodiesel
- Electrolyte in alkaline batteries
- Chemical manufacturing
- Calcium Hydroxide (Ca(OH)₂)
- Water treatment
- Soil stabilization
- Mortar and plaster in construction
- Barium Hydroxide (Ba(OH)₂)
- Laboratory reagent
- Manufacturing of thermoplastics
- Removing sulfate ions from solutions
- Lithium Hydroxide (LiOH)
- Electrolyte in lithium-ion batteries
- Air purification systems (removes CO₂)
- Production of ceramics and glass
pH and pOH Concepts
pH
The pH of a solution is a measure of its acidity or hydrogen ion concentration [H⁺]. It is calculated using the formula: pH = −log[H⁺]
- Acidic Solutions: pH less than 7
- Neutral Solutions: pH equal to 7
- Basic (Alkaline) Solutions: pH greater than 7
pOH
The pOH of a solution is a measure of its basicity or hydroxide ion concentration [OH⁻]. It is calculated using the formula: pOH = −log[OH⁻]
- Basic (Alkaline) Solutions: pOH less than 7
- Neutral Solutions: pOH equal to 7
- Acidic Solutions: pOH greater than 7
Relationship between pH and pOH
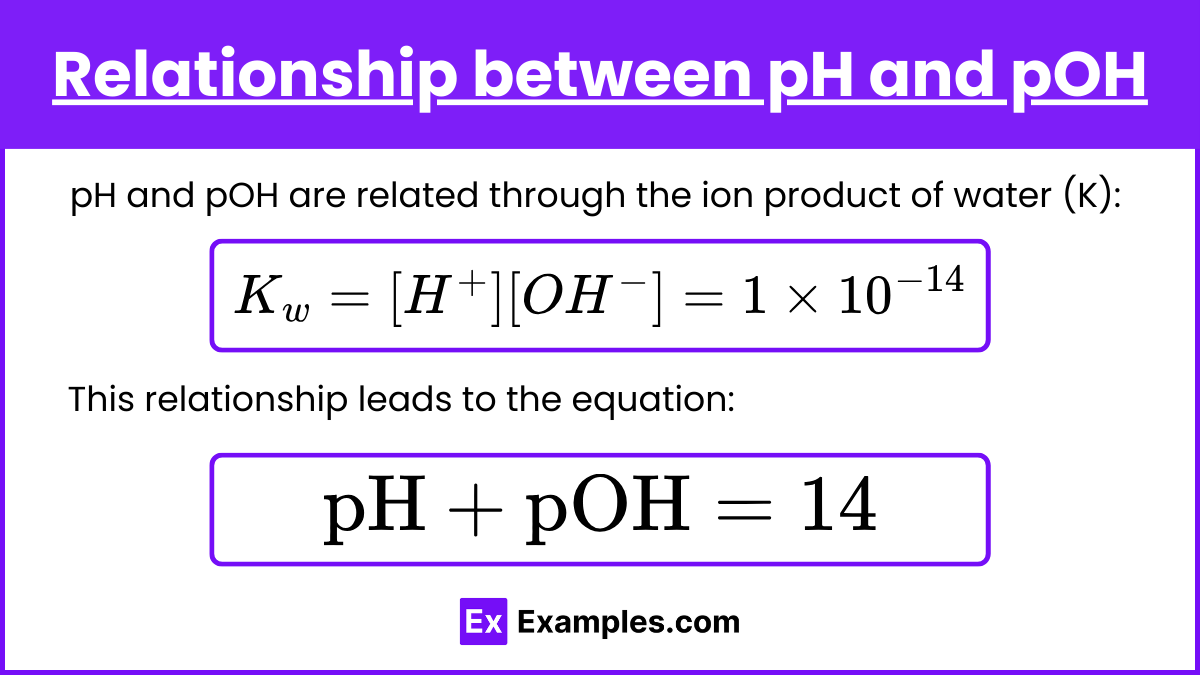
pH and pOH are related through the ion product of water (K):
Kw = [H⁺][OH⁻] = 1 ×10⁻¹⁴
This relationship leads to the equation:
pH + pOH = 14
Practice Problems
1. Calculating pH of a Strong Acid
Question: Calculate the pH of a 0.02 M HNO₃ solution.
Solution:
- Determine [H⁺]: [H⁺] = 0.02 M
- Calculate pH: pH = −log(0.02)
- pH = 1.7
2. Calculating pH of a Strong Base
Question: Calculate the pH of a 0.05 M KOH solution.
Solution:
- Determine [OH⁻]: [OH⁻] = 0.05 M
- Calculate pOH: pOH = −log(0.05)
- pOH = 1.3
- Calculate pH: pH = 14 − pOH
- pH = 14 − 1.3
- pH = 12.7
3. Calculating pOH of a Strong Acid
Question: Calculate the pOH of a 0.01 M HCl solution.
Solution:
- Determine [H⁺]: [H⁺] = 0.01 M
- Calculate pH: pH = −log(0.01)
- pH = 2
- Calculate pOH: pOH = 14−pH
- pOH = 14 − 2
- pOH = 12
4. Calculating pH of a Strong Base with Polyvalent Ions
Question: Calculate the pH of a 0.02 M Ba(OH)₂ solution.
Solution:
- Determine [OH⁻]:
- Barium hydroxide dissociates completely and releases two hydroxide ions per formula unit:
- [OH⁻] = 2 × 0.02 M=0.04 M
- Calculate pOH: pOH = −log(0.04)
- pOH =1.4
- Calculate pH: pH = 14 − pOH
- pH=14−1.4
- pH=12.6