Learning Objectives
For the AP Chemistry exam, understand how to calculate and interpret pH for various solutions, including strong and weak acids, bases, and salts. Master the pH scale, solubility product constant (Kₛₚ), and factors affecting solubility, such as temperature, pressure, and the common ion effect. Learn to predict precipitation, construct and interpret titration curves, and understand buffer solutions using the Henderson-Hasselbalch equation. Focus on practical applications to enhance your problem-solving skills in these areas.
Free AP Chemistry Practice Test
Introduction
pH measures the acidity or basicity of a solution on a scale from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating basicity. The pH scale is logarithmic, meaning each unit change represents a tenfold difference in acidity or basicity. Solubility refers to a substance's ability to dissolve in a solvent, forming a solution, and is influenced by the nature of the solute and solvent, temperature, and pressure. The pH can significantly impact solubility, especially for substances that are weak acids or bases, where acidic conditions can increase the solubility of basic compounds and vice versa. Understanding these concepts is essential in chemistry and various scientific fields for predicting reactions and controlling processes.
pH
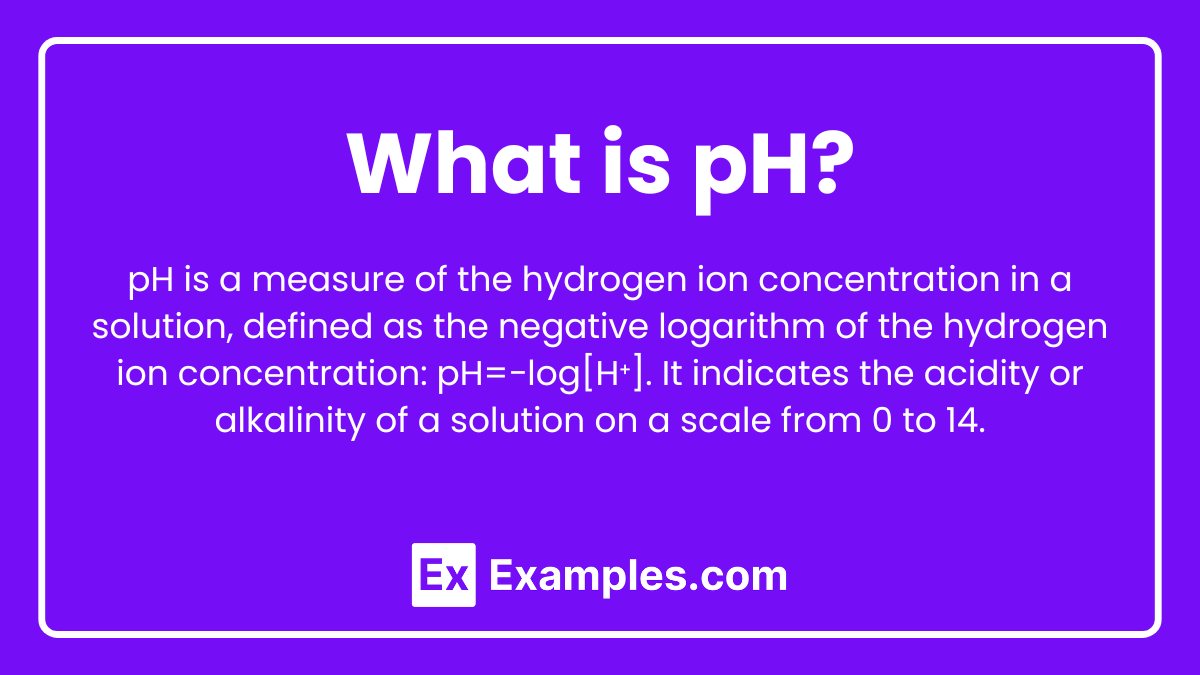
What is pH?
pH is a scale used to specify the acidity or basicity of an aqueous solution. It is defined as the negative logarithm of the hydrogen ion concentration in a solution, expressed as:
pH = −log[H⁺]
where [H⁺] is the concentration of hydrogen ions in moles per liter. The pH scale ranges from 0 to 14, with 7 being neutral, values less than 7 indicating acidity, and values greater than 7 indicating basicity.
pH Scale
The pH scale is a numerical scale used to measure the acidity or basicity of an aqueous solution. It ranges from 0 to 14, and is based on the concentration of hydrogen ions [H⁺] in the solution.
Key Points of the pH Scale:
Neutral (pH 7): A pH of 7 indicates a neutral solution, where the concentration of hydrogen ions equals the concentration of hydroxide ions [OH⁻]. Pure water is an example of a neutral substance.
Acidic (pH < 7): Solutions with a pH less than 7 are acidic. The lower the pH, the higher the concentration of hydrogen ions. Examples include lemon juice and vinegar.
Basic (pH > 7): Solutions with a pH greater than 7 are basic (or alkaline). The higher the pH, the lower the concentration of hydrogen ions. Examples include baking soda and soap.
Logarithmic Nature of the pH Scale:
The pH scale is logarithmic, which means each whole number change on the scale represents a tenfold change in hydrogen ion concentration. For instance:
A solution with a pH of 4 has ten times more hydrogen ions than a solution with a pH of 5.
A solution with a pH of 9 has one-tenth the hydrogen ion concentration of a solution with a pH of 8.
Calculating pH
Calculating the pH of a solution involves determining the concentration of hydrogen ions [H+][H^+][H+] in the solution and then using the pH formula. Here's a step-by-step guide on how to calculate pH:
pH Formula
pH = −log[H⁺]
where [H⁺] is the concentration of hydrogen ions in moles per liter (M).
Steps to Calculate pH:
Determine the Hydrogen Ion Concentration:
Measure or obtain the concentration of hydrogen ions [H⁺] in the solution. This is typically given in moles per liter (M).
Apply the pH Formula:
Use the formula pH = −log[H⁺] to calculate the pH.
pH of Salt Solutions
Salt solutions can have varying pH levels depending on the nature of the salt. When salts dissolve in water, they can produce solutions that are acidic, neutral, or basic. This behavior is determined by the ions that make up the salt and how they interact with water.
Types of Salt Solutions
Neutral Salts:
Example: Sodium chloride (NaCl)
When neutral salts dissolve in water, they do not affect the pH significantly.
Reaction: NaCl → Na⁺ + Cl⁻
Both Na⁺ and Cl⁻ ions do not react with water to form H⁺ or OH⁻ ions.
Acidic Salts:
Example: Ammonium chloride (NH Cl)
Acidic salts come from a strong acid and a weak base.
Reaction: NH₄Cl → NH₄⁺ + Cl⁻
The NH4⁺ ion reacts with water to produce H⁺ : NH₄⁺ + H₂O → NH₃+ H₃O⁺
This increases the concentration of H⁺ in the solution, lowering the pH.
Basic Salts:
Example: Sodium acetate (CH₃COONa)
Basic salts come from a weak acid and a strong base.
Reaction: CH₃COONa → CH₃COO⁻ + Na⁺
The CH₃COO⁻ ion reacts with water to produce OH⁻: CH₃COO⁻ + H₂O →CH₃COOH + OH⁻
This increases the concentration of OH⁻ in the solution, raising the pH.
Determining pH of Salt Solutions:
Identify the Salt:
Determine the cation (positive ion) and anion (negative ion) from the salt.
Determine the Parent Acid and Base:
Identify the acid and base that would combine to form the salt.
Evaluate the Strengths of the Acid and Base:
Strong acid + strong base → Neutral salt (e.g., NaCl).
Strong acid + weak base → Acidic salt .
Weak acid + strong base → Basic salt .
Solubility
What is Solubility?
Solubility is defined as the maximum amount of a solute that can dissolve in a given quantity of solvent at a specific temperature and pressure, resulting in a saturated solution. It is typically expressed in units of grams of solute per 100 grams of solvent (g/100g) or moles of solute per liter of solution (mol/L).
Solubility Product Constant (Kₛₚ)
The Solubility Product Constant (Kₛₚ) is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the product of the molar concentrations of the constituent ions, each raised to the power of their stoichiometric coefficients, in a saturated solution at a given temperature.
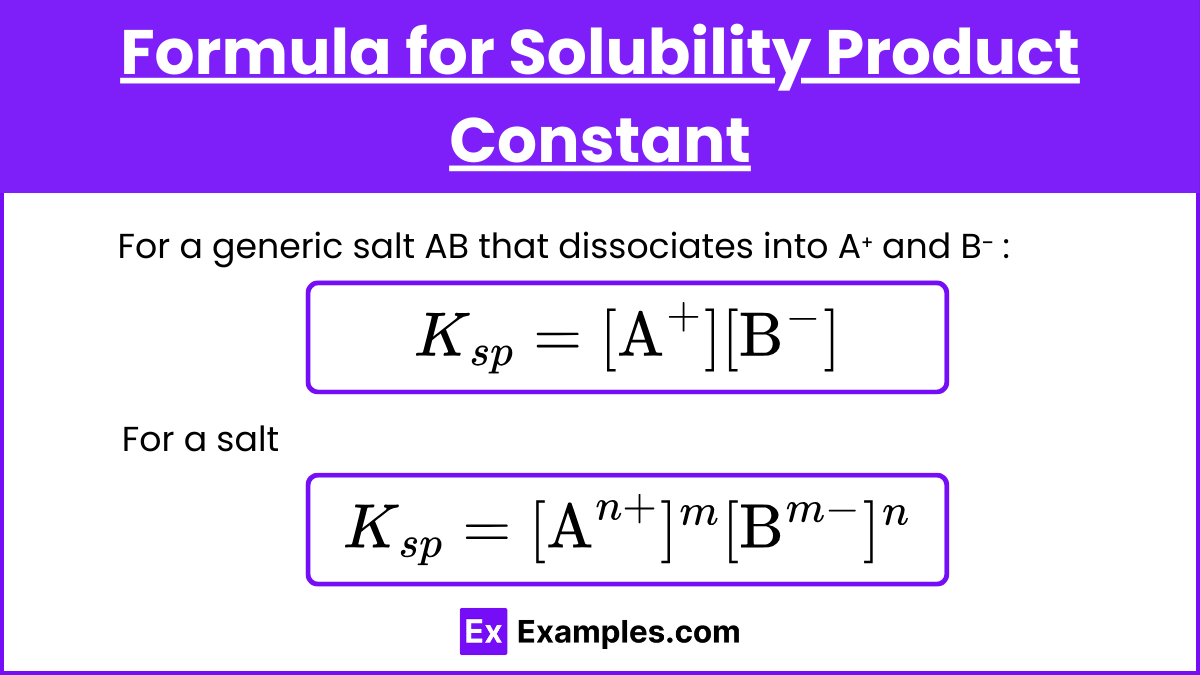
Formula for Solubility Product Constant (Kₛₚ)
For a generic salt AB that dissociates into A⁺ and B⁻ :
AB ⇌ A⁺ + B⁻
Kₛₚ = [A⁺][B⁻]
For a salt
AₘBₙ ⇌ mAⁿ⁺+nBᵐ⁻
Kₛₚ=[Aⁿ⁺]ᵐ[Bᵐ⁻]ⁿ
Factors Affecting Solubility
Nature of the Solute and Solvent
Polar solutes dissolve in polar solvents.
Nonpolar solutes dissolve in nonpolar solvents.
Temperature
Solids in liquids: Solubility generally increases with temperature.
Gases in liquids: Solubility decreases with increasing temperature.
Pressure
Primarily affects the solubility of gases.
Solubility of gases in liquids increases with increasing pressure (Henry's Law).
Common Ion Effect
Presence of a common ion decreases the solubility of a salt.
Example: Adding NaCl to a solution of AgCl decreases AgCl solubility.
pH of the Solution
Affects the solubility of salts with basic or acidic ions.
Example: Solubility of CaCO₃ increases in acidic solutions due to the reaction with H⁺ ions.
Presence of Complexing Agents
Complexing agents can increase the solubility of a salt by forming soluble complexes.
Example: AgCl solubility increases in the presence of ammonia due to the formation of Ag(NH₃)₂⁺.
Solvent Polarity
Solubility varies depending on the polarity of the solvent.
Polar solutes are more soluble in polar solvents, while nonpolar solutes are more soluble in nonpolar solvents.
Buffer Solutions
A buffer solution is a solution that resists changes in pH when small amounts of an acid or a base are added. Buffers maintain a relatively constant pH, making them crucial in many chemical and biological processes.
Components
A typical buffer solution consists of:
A weak acid and its conjugate base (e.g., acetic acid and sodium acetate).
A weak base and its conjugate acid (e.g., ammonia and ammonium chloride).
Buffer Action
Buffers work by neutralizing added acids or bases. When an acid (H⁺) is added to a c, the conjugate base neutralizes it. Conversely, when a base (OH⁻) is added, the weak acid neutralizes it.
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation relates the pH of a buffer solution to the concentration of the weak acid and its conjugate base:
Where:
pH is the measure of acidity.
pKₐ is the negative logarithm of the acid dissociation constant Kₐ.
A⁻ is the concentration of the conjugate base.
HA is the concentration of the weak acid.
How Buffers Work
Buffer with Weak Acid and Conjugate Base
Example: Acetic acid CH₃COOH and sodium acetate (CH₃COONa).
When H⁺ is added: CH₃COO⁻ + H⁺ → CH₃COOH.
When OH⁻ is added: CH₃COOH + OH⁻→ CH₃COO⁻ + H2O.
Buffer with Weak Base and Conjugate Acid
Example: Ammonia (NH₃) and ammonium chloride (NH₄Cl).
When H⁺ is added: .
When OH⁻ is added: .
Importance of Buffers
Biological Systems: Maintain the pH of blood and cellular fluids.
Chemical Reactions: Provide a stable pH environment for reactions.
Industrial Processes: Used in fermentation, pharmaceuticals, and food production.
Precipitation
Precipitation occurs when the product of the ionic concentrations in a solution exceeds the Kₛₚ of the compound, resulting in the formation of a solid.
Predicting Precipitation
To predict whether a precipitate will form, calculate the ion product (Q) and compare it to Kₛₚ:
If Q<Kₛₚ, the solution is unsaturated, and no precipitate forms.
If Q=Kₛₚ, the solution is saturated, and no change occurs.
If Q>Kₛₚ, the solution is supersaturated, and a precipitate forms.
Ion Product (Q)
The ion product (Q) is calculated using the same expression as K, but with the actual concentrations of the ions in the solution.
Factors Affecting Solubility and Precipitation
Common Ion Effect: The solubility of a salt decreases in the presence of a common ion.
pH of the Solution: The solubility of salts with basic anions increases in acidic solutions.
Complexation: Complexing agents can increase solubility by forming soluble complexes.
Solubility of Salts in Acidic Solutions
The solubility of salts can be significantly affected by the pH of the solution. In acidic solutions, the presence of hydrogen ions (H⁺) can alter the solubility of salts, especially those containing basic anions.
Mechanism
Salts with Basic Anions:
Salts that contain anions of weak acids (such as increase in solubility in acidic solutions.
The ions from the acidic solution react with the basic anions, reducing their concentration and shifting the equilibrium to dissolve more salt.
General Rule
Acidic solutions increase the solubility of salts with basic anions: Salts like , and others become more soluble in the presence of acid because the acidic environment neutralizes the basic anions.
Common Ion Effect on Solubility
The common ion effect refers to the decrease in solubility of an ionic compound when a common ion is added to the solution. This phenomenon is a direct consequence of Le Chatelier's Principle, which states that the equilibrium will shift to counteract the change imposed on the system.
Mechanism
When a common ion is added to a saturated solution of a sparingly soluble salt, the equilibrium shifts to reduce the solubility of the salt. This is because the increased concentration of the common ion suppresses the dissociation of the salt.
Factors Affecting the Common Ion Effect
Concentration of the Common Ion:
Higher concentrations of the common ion lead to a greater decrease in solubility.
Nature of the Salt:
The extent of the common ion effect depends on the solubility product (Kₛₚ) of the salt.