Learning Objectives
By the end of this lesson, you should be able to understand the principles of Photoelectron Spectroscopy (PES) and explain the photoelectric effect. You will learn to describe the instrumentation and processes involved in PES, including how to prepare samples and analyze data. You should be able to interpret PES spectra to determine the binding energies of electrons and identify peaks corresponding to different atomic or molecular orbitals. Additionally, you will explore the applications of PES in atomic and molecular structure determination and chemical analysis, and solve related problems by calculating binding energies and interpreting spectra.
Free AP Chemistry Practice Test
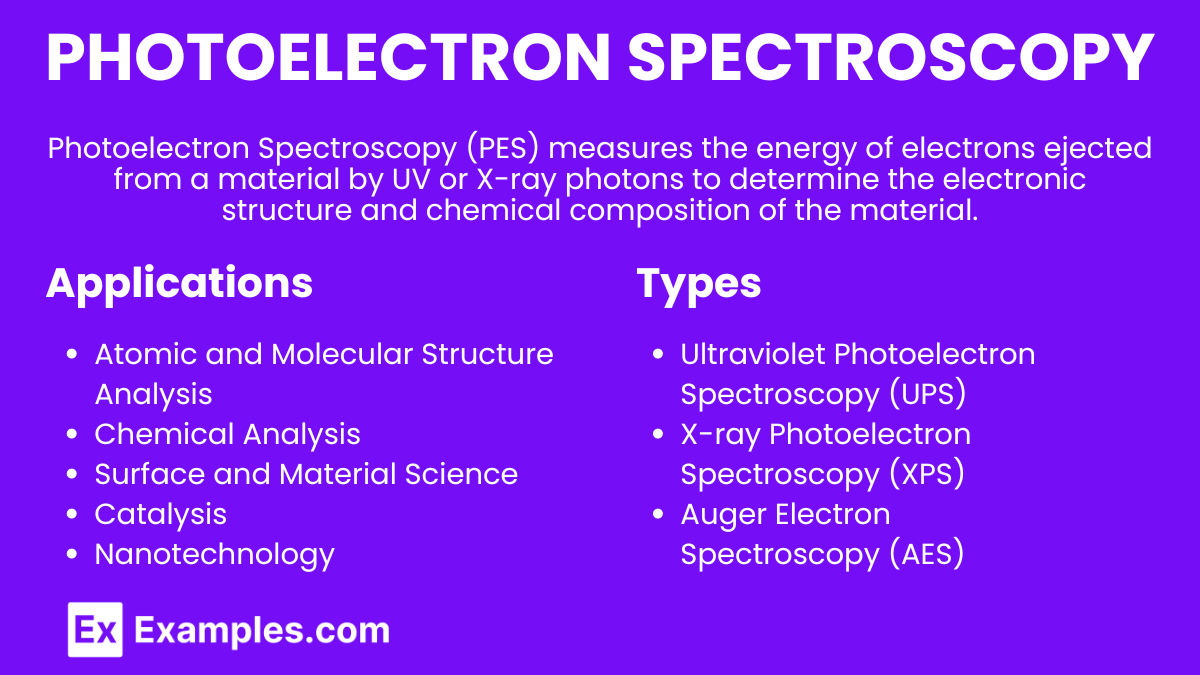
Introduction to Photoelectron Spectroscopy (PES)
Photoelectron Spectroscopy (PES) is a technique used to study the electronic structure of atoms and molecules. By analyzing the energy of electrons emitted from a substance when it is exposed to ultraviolet (UV) or X-ray photons, we can determine the binding energies of electrons in different atomic or molecular orbitals.
What is Photoelectron Spectroscopy?
Photoelectron Spectroscopy (PES) is an analytical technique used to determine the electronic structure of atoms and molecules by irradiating a sample with high-energy photons (ultraviolet or X-rays) and measuring the kinetic energy of emitted electrons. This method provides information about the binding energies of electrons in different orbitals, revealing details about the elemental composition, chemical states, and electronic configurations of the sample.
Types of Photoelectron Spectroscopy
Ultraviolet Photoelectron Spectroscopy (UPS)
Ultraviolet Photoelectron Spectroscopy (UPS) uses ultraviolet (UV) photons, typically in the energy range of 10-200 eV, to eject electrons from the outermost orbitals of atoms or molecules. UPS is primarily used to study valence electrons, which are important for understanding the chemical bonding and electronic properties of a material. This technique is particularly useful in surface science, as it provides information about the electronic structure and chemical states of surfaces and thin films.
X-ray Photoelectron Spectroscopy (XPS)
X-ray Photoelectron Spectroscopy (XPS) employs X-ray photons, usually with energies between 200 and 1500 eV, to eject electrons from both core and valence orbitals. XPS is widely used for elemental analysis and chemical state identification because core electrons are tightly bound and their binding energies are highly element-specific. This technique is essential in materials science, chemistry, and surface analysis, as it allows for precise determination of the elemental composition and oxidation states of materials.
Auger Electron Spectroscopy (AES)
While not a direct type of PES, Auger Electron Spectroscopy (AES) is often related and used in conjunction with XPS. AES involves the ejection of Auger electrons as a result of a relaxation process following the ionization of core electrons. It provides complementary information to XPS, especially useful for surface analysis and detecting light elements. AES is highly surface-sensitive and is commonly used to study the elemental composition and chemical states at surfaces and interfaces.
Principles of Photoelectron Spectroscopy
The Photoelectric Effect
Photoelectron Spectroscopy is based on the photoelectric effect, which occurs when photons with sufficient energy strike a material, causing the ejection of electrons. Albert Einstein explained this effect, stating that when photons hit a material, they transfer their energy to electrons. If this energy exceeds the binding energy of the electrons, they are emitted from the material. The relationship between the energy of the incident photons (hv), the kinetic energy of the emitted electrons (KE), and the binding energy (BE) of the electrons is given by the equation:
KE = hv − BE
KE: Kinetic energy of the emitted electron
hv: Energy of the incident photon
BE: Binding energy of the electron
Binding Energy
The binding energy is the energy required to remove an electron from an atom or molecule. It is a crucial parameter in PES as it provides insights into the electronic structure and the chemical environment of the atoms in a sample. Different orbitals have characteristic binding energies, allowing identification of specific elements and their electronic configurations. Core electrons, which are closer to the nucleus, have higher binding energies compared to valence electrons.
Energy Conservation
In PES, the conservation of energy principle is fundamental. The energy of the incident photon is conserved by being partitioned between the kinetic energy of the emitted electron and its binding energy. By measuring the kinetic energy of the emitted electrons, one can calculate their binding energies, thus obtaining information about the electronic structure of the sample.
Instrumentation and Process
Sample Preparation: The sample can be solid, liquid, or gas.
Photon Source: UV or X-ray light source to bombard the sample.
Electron Analyzer: Detects the kinetic energy of ejected electrons.
Data Analysis: Kinetic energy is converted to binding energy using the equation:
E_{binding} = E_{photon} - E_{kinetic}
Interpreting PES Data
Binding Energy Spectrum
The PES spectrum displays peaks corresponding to electrons ejected from different orbitals.
Peak Position: Indicates the binding energy of electrons.
Peak Height/Area: Proportional to the number of electrons in that orbital.
Orbital Identification
Core Electrons: High binding energy peaks (e.g., 1s, 2s).
Valence Electrons: Lower binding energy peaks (e.g., 2p, 3s).
Applications of Photoelectron Spectroscopy
Atomic and Molecular Structure Analysis
Electronic Structure: Determines the distribution of electrons in orbitals.
Ionization Energies: Measures energies essential for understanding reactivity and bonding.
Chemical Analysis
Elemental Composition: Identifies elements in a sample.
Chemical and Oxidation States: Differentiates between chemical states (e.g., Fe²⁺ vs. Fe³⁺).
Surface and Material Science
Surface Composition: Analyzes the outermost layers of materials.
Interface Analysis: Investigates interfaces between different materials.
Catalysis
Catalyst Characterization: Studies electronic structure and chemical states of catalysts.
Reaction Mechanisms: Provides insights into how catalysts interact with reactants.
Nanotechnology
Nanoparticle Analysis: Characterizes electronic structure and surface composition of nanoparticles.
Quantum Dots and Nanostructures: Studies electronic properties important for optoelectronics.
Environmental Science
Pollutant Analysis: Detects and analyzes surface pollutants and their chemical states.
Aerosol Analysis: Studies composition and states of atmospheric particulate matter.
Semiconductor Industry
Doping and Defects: Characterizes doping levels and defects in semiconductors.
Band Structure Analysis: Provides information on electronic properties of semiconductors.
Case Study: Graphene
Electronic Properties: Studies unique properties like Dirac cones and band structure.
Surface Functionalization: Analyzes changes in surface chemistry with different treatments.
Practice Problems
PES Spectrum of Oxygen
Consider a PES spectrum of an oxygen atom:
Orbital | Binding Energy (eV) | Relative Peak Intensity |
|---|---|---|
1s | 543 | Medium |
2s | 41 | Small |
2p | 13 | Large |
The 1s orbital peak at 543 eV represents core electrons with a medium intensity.
The 2s orbital peak at 41 eV represents core electrons with a small intensity.
The 2p orbital peak at 13 eV represents valence electrons with a large intensity.
PES Spectrum of Nitrogen
Consider a PES spectrum of a nitrogen atom:
Orbital | Binding Energy (eV) | Relative Peak Intensity |
|---|---|---|
1s | 410 | Medium |
2s | 36 | Small |
2p | 15 | Large |
The 1s orbital peak at 410 eV represents core electrons with a medium intensity.
The 2s orbital peak at 36 eV represents core electrons with a small intensity.
The 2p orbital peak at 15 eV represents valence electrons with a large intensity.
PES Spectrum of Silicon
Consider a PES spectrum of a silicon atom:
Orbital | Binding Energy (eV) | Relative Peak Intensity |
|---|---|---|
1s | 1840 | Medium |
2s | 150 | Small |
2p | 99 | Large |
The 1s orbital peak at 1840 eV represents core electrons with a medium intensity.
The 2s orbital peak at 150 eV represents core electrons with a small intensity.
The 2p orbital peak at 99 eV represents valence electrons with a large intensity.