Learning Objectives
By the end of this lesson, AP Chemistry students should be able to distinguish between physical and chemical changes, describe the characteristics and examples of each, understand the molecular-level differences between physical and chemical changes, and apply their knowledge to predict and explain changes observed in various substances. Additionally, students should be able to identify signs of chemical changes, understand the conservation of mass during these changes, and relate these concepts to real-world examples and laboratory experiments.
Introduction
Physical and chemical changes are fundamental concepts in chemistry that describe how substances undergo alterations. A physical change involves a change in the physical properties of a substance, such as its shape, state, or appearance, without altering its chemical composition. Examples include melting ice, tearing paper, and dissolving sugar in water. In contrast, a chemical change results in the formation of one or more new substances with different chemical properties and compositions, such as when iron rusts, wood burns, or milk sours. Understanding these changes is crucial for comprehending various chemical processes and reactions in both everyday life and industrial applications.
Physical Changes
What are Physical Changes?
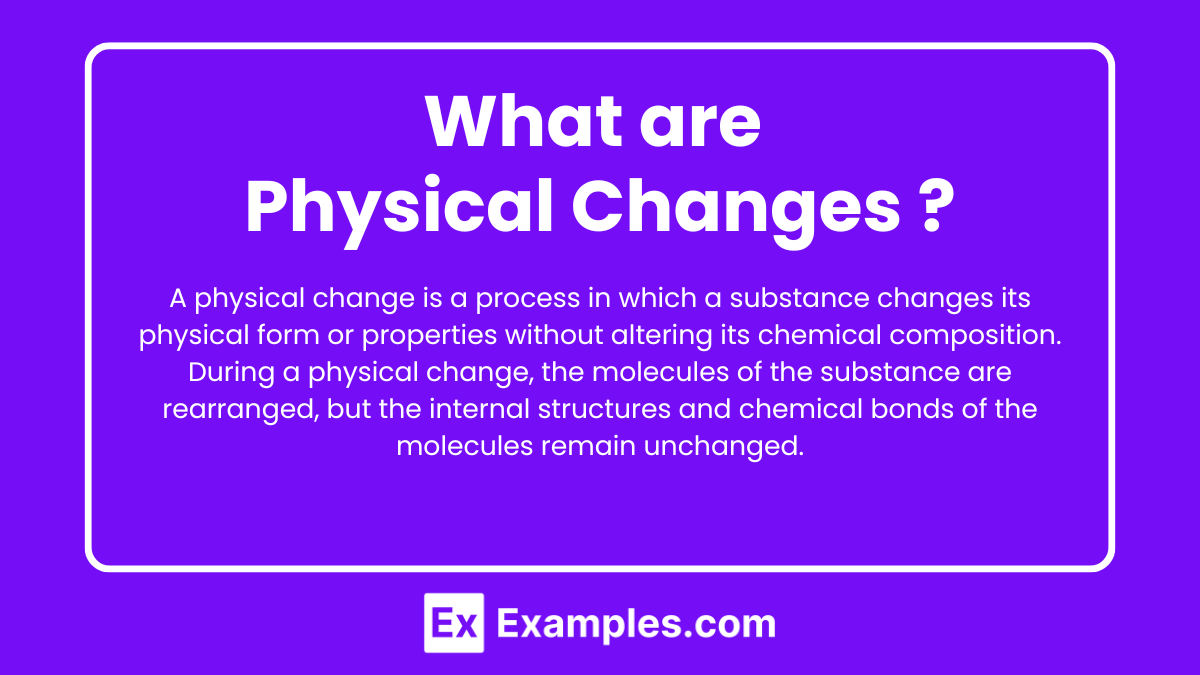
A physical change is a process in which a substance changes its physical form or properties without altering its chemical composition. During a physical change, the molecules of the substance are rearranged, but the internal structures and chemical bonds of the molecules remain unchanged.
Characteristics of Physical Changes
- No New Substance Formed
- Reversible Changes
- Most physical changes can be reversed by changing the conditions back to their original state. This means that the substance can return to its initial form. For instance, water can be frozen back into ice by lowering the temperature again.
- Energy Change
- Physical changes typically involve the absorption or release of energy, usually in the form of heat. However, these energy changes do not affect the chemical bonds within the molecules. For example, heating water causes it to boil, while cooling it causes it to freeze, but in both cases, the molecular structure of water remains the same.
- Change in State or Form
- Physical changes often involve a transition between different states of matter (solid, liquid, gas) or alterations in the form or appearance of a substance. This does not change the chemical composition of the substance. For example, bending a metal rod changes its shape but not its chemical properties.
- No Change in Molecular Structure
- The internal molecular structure of the substance remains unchanged during a physical change. The molecules may move closer together or farther apart, but their individual structures stay the same. For example, when water evaporates, the H₂O molecules spread out in the gaseous state but remain intact.
- Physical Property Alteration
- Physical changes result in alterations to the physical properties of a substance such as size, shape, color, texture, and phase. These changes are often visible and can be measured without changing the chemical identity of the substance. For example, grinding a crystal of salt changes its size and texture, but it is still chemically salt.
Molecular-Level Explanation
Physical changes involve changes in the physical properties or state of a substance without altering its chemical structure.
Molecular Arrangement
In physical changes, the arrangement or distance between molecules changes, but the molecules themselves remain intact. This can involve changes in state (solid, liquid, gas) or other physical properties such as shape or size.
Types of Molecular Movement
- Solids: In a solid, molecules are closely packed in a fixed, orderly arrangement. They vibrate but do not move freely.
- Liquids: In a liquid, molecules are still close together but can move past each other, allowing the liquid to flow.
- Gases: In a gas, molecules are far apart and move freely at high speeds.
Examples of Molecular-Level Changes
- Melting (Solid to Liquid):
- When a solid melts, the molecules gain energy (usually from heat) and start to vibrate more vigorously.
- As the temperature rises, these vibrations become strong enough to overcome the forces holding the molecules in their fixed positions.
- The molecules then move past each other, transitioning into a liquid state.
- Boiling (Liquid to Gas):
- When a liquid boils, the molecules gain enough energy to break free from the liquid’s surface and enter the gas phase.
- In this process, the intermolecular forces (such as hydrogen bonds in water) are overcome by the kinetic energy of the molecules.
- Freezing (Liquid to Solid):
- When a liquid freezes, the molecules lose energy and slow down.
- As the temperature drops, the molecules arrange themselves into a fixed, orderly pattern, forming a solid.
- The attractive forces between the molecules become strong enough to hold them in place.
- Dissolving:
- When a substance dissolves, the molecules of the solute spread out and interact with the molecules of the solvent.
- For example, when salt (NaCl) dissolves in water, the Na⁺ and Cl⁻ ions separate and become surrounded by water molecules.
- The ionic bonds in the salt are broken, but no new chemical substances are formed.
Conservation of Mass
- In physical changes, the mass of the substance remains constant.
- The number and type of atoms in the molecules do not change, ensuring that the substance’s chemical identity is preserved.
Energy Changes
- Energy changes in physical processes usually involve changes in the kinetic energy of the molecules.
- During melting and boiling, energy is absorbed to overcome intermolecular forces.
- During freezing and condensation, energy is released as molecules slow down and form stronger intermolecular attractions.
Chemical Changes
What are Chemical Changes?
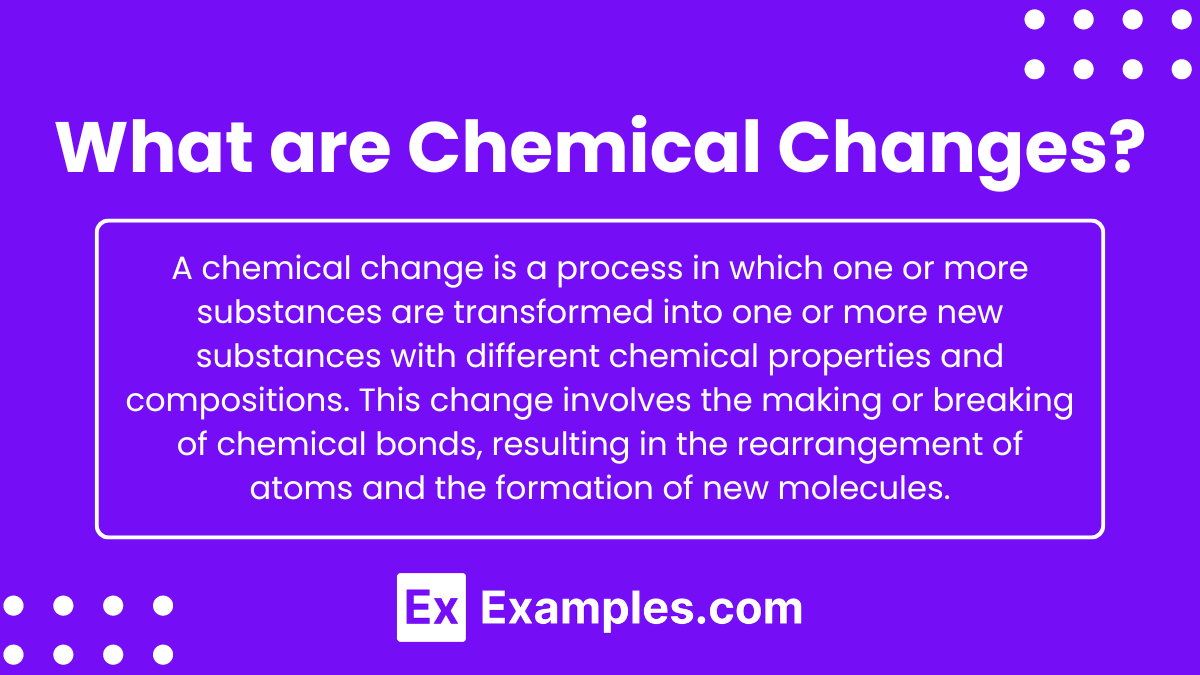
A chemical change is a process in which one or more substances are transformed into one or more new substances with different chemical properties and compositions. This change involves the making or breaking of chemical bonds, resulting in the rearrangement of atoms and the formation of new molecules.
Characteristics of Chemical Changes
- Formation of New Substances
- Chemical changes result in the creation of one or more new substances with different chemical compositions and properties from the original substances. This occurs because chemical bonds are broken and new bonds are formed during the reaction.
- Irreversibility
- Most chemical changes are not easily reversible through simple physical means. Returning to the original substances typically requires another chemical reaction. For example, burning wood produces ash and gases that cannot be reverted back to wood.
- Energy Change
- Chemical changes often involve significant changes in energy. Energy may be released (exothermic reactions) or absorbed (endothermic reactions) during the process. This energy change is due to the breaking and formation of chemical bonds.
- Color Change
- A change in color is often a sign that a chemical change has occurred. This happens because the new substances formed may have different colors compared to the reactants.
- Gas Production
- The production of a gas is a common indicator of a chemical change. This can be observed as bubbles forming in a liquid, the release of fumes, or the formation of a gas cloud.
- Precipitate Formation
- In some chemical reactions, a solid (precipitate) forms from the reaction of two liquids or a liquid and a gas. The precipitate indicates the formation of a new substance.
- Odor Change
- A change in odor can indicate a chemical change as new substances with different smells are produced. For example, rotting food produces different odors than fresh food due to chemical changes.
- Temperature Change
- A noticeable change in temperature, without the addition of external heat, suggests a chemical reaction is taking place. This is due to the release or absorption of energy during the reaction.
Molecular-Level Explanation
Chemical changes involve the breaking and forming of chemical bonds between atoms, resulting in the creation of new substances with different chemical properties and compositions.
Breaking and Forming Bonds
- Reactants to Products: In a chemical change, the original substances (reactants) undergo a transformation where their atoms are rearranged to form new substances (products). This rearrangement involves breaking existing chemical bonds and forming new ones.
- Bond Energy: The energy required to break bonds and the energy released when new bonds are formed play a crucial role in chemical reactions. The difference in these energy changes determines whether a reaction is exothermic (releases energy) or endothermic (absorbs energy).
Example: Combustion of Methane
- Reactants: Methane (CH₄) and Oxygen (O₂).
- Bond Breaking: The C-H bonds in methane and the O=O bonds in oxygen molecules are broken.
- Atom Rearrangement: The carbon (C) and hydrogen (H) atoms from methane combine with oxygen (O) atoms.
- New Bonds Forming: New C=O bonds form in carbon dioxide (CO₂) and O-H bonds form in water (H₂O).
- Products: The chemical change results in the formation of carbon dioxide (CO₂) and water (H₂O), which are chemically different from the reactants.
Conservation of Mass
- Atomic Count: The number of each type of atom remains the same before and after the reaction, adhering to the law of conservation of mass. Atoms are neither created nor destroyed; they are simply rearranged.
- Balanced Equations: Chemical equations must be balanced to reflect this conservation, showing the same number of each type of atom on both sides of the equation.
Energy Changes
- Exothermic Reactions: When the energy released from forming new bonds is greater than the energy required to break the initial bonds, the reaction releases energy, often in the form of heat or light.
- Endothermic Reactions: When more energy is required to break the initial bonds than is released from forming new bonds, the reaction absorbs energy, often resulting in a drop in temperature.
Electron Transfer
- Redox Reactions: In some chemical changes, electrons are transferred between atoms. Oxidation involves the loss of electrons, while reduction involves the gain of electrons. Redox reactions are fundamental to processes like corrosion, respiration, and photosynthesis.
Molecular-Level Changes Examples
- Rusting of Iron:
- Reactants: Iron (Fe) and Oxygen (O₂).
- Bond Breaking: Fe bonds in iron and O=O bonds in oxygen.
- New Bonds: Formation of Fe₂O₃ (iron oxide) bonds.
- Products: Rust (Fe₂O₃) forms, with distinct properties from iron.
- Photosynthesis:
- Reactants: Carbon dioxide (CO₂) and water (H₂O).
- Bond Breaking: C=O bonds in CO₂ and O-H bonds in H₂O.
- New Bonds: Formation of C-H bonds in glucose (C₆H₁₂O₆) and O=O bonds in oxygen (O₂).
- Products: Glucose and oxygen, which are chemically different from the reactants.
Comparing Physical and Chemical Changes
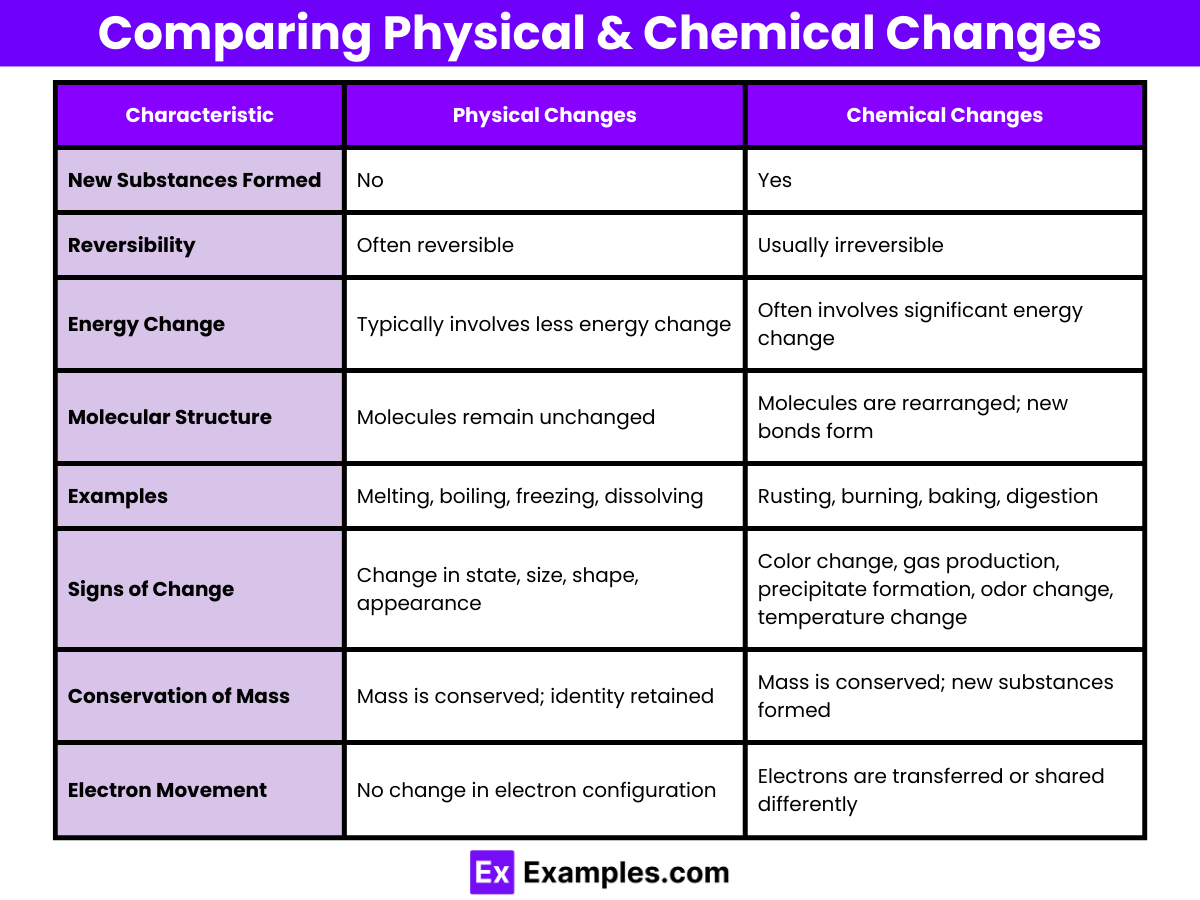
| Characteristic | Physical Changes | Chemical Changes |
|---|---|---|
| New Substances Formed | No | Yes |
| Reversibility | Often reversible | Usually irreversible |
| Energy Change | Typically involves less energy change | Often involves significant energy change |
| Molecular Structure | Molecules remain unchanged | Molecules are rearranged; new bonds form |
| Examples | Melting, boiling, freezing, dissolving | Rusting, burning, baking, digestion |
| Signs of Change | Change in state, size, shape, appearance | Color change, gas production, precipitate formation, odor change, temperature change |
| Conservation of Mass | Mass is conserved; identity retained | Mass is conserved; new substances formed |
| Electron Movement | No change in electron configuration | Electrons are transferred or shared differently |