Learning Objectives
By studying the properties of buffers for the AP Chemistry exam, you should aim to understand the definition and function of buffers, including how they resist changes in pH upon the addition of acids or bases. You should be able to apply the Henderson-Hasselbalch equation to calculate the pH of buffer solutions and determine the appropriate acid-base pair for a desired pH range. Additionally, you should comprehend the concepts of buffer capacity and buffer range, recognize common buffer systems (e.g., acetic acid/acetate, phosphate buffer, carbonate buffer), and understand their applications in biological and chemical systems. Mastering these objectives will prepare you to solve buffer-related problems and explain their significance in various contexts.
Introduction
Buffers are solutions that resist changes in pH when small amounts of acids or bases are added. They are crucial in maintaining the pH levels in biological and chemical systems. Buffers typically consist of a weak acid and its conjugate base or a weak base and its conjugate acid. Understanding the properties of buffers helps in various applications, including biochemical processes, pharmaceuticals, and industrial chemistry. Key properties of buffers include their ability to maintain pH stability, buffer capacity, and the pH range in which they are effective.
What is Buffer?
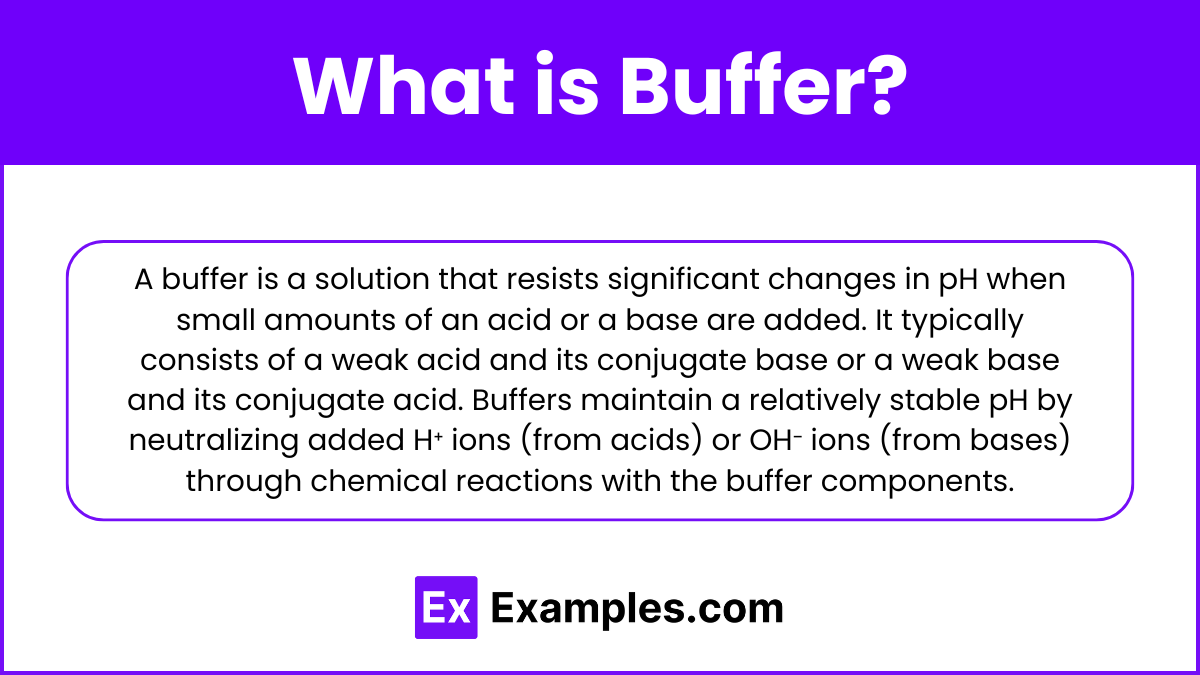
A buffer is a solution that resists significant changes in pH when small amounts of an acid or a base are added. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers maintain a relatively stable pH by neutralizing added H⁺ ions (from acids) or OH⁻ ions (from bases) through chemical reactions with the buffer components.
How Buffer Works?
Buffers work by neutralizing added acids (H⁺ ions) or bases (OH⁻ ions) through reactions with the components of the buffer solution. This process helps maintain a stable pH in the solution.
Components of a Buffer
A buffer typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Mechanism of Action
- Addition of Acid (H⁺ ions):
- When an acid is added to the buffer solution, the added H⁺ ions are neutralized by the conjugate base present in the buffer.
- Example: In an acetic acid/sodium acetate buffer: CH₃COO⁻ + H⁺ → CH₃COOH
- Here, acetate ions (CH₃COO⁻) react with the added H⁺ ions to form acetic acid (CH₃COOH), thereby minimizing the increase in pH.
- Addition of Base (OH⁻ ions):
- When a base is added, the added OH⁻ ions are neutralized by the weak acid in the buffer.
- Example: In an acetic acid/sodium acetate buffer: CH₃COOH + OH⁻ → CH₃COO⁻ + H₂O
- Here, acetic acid (CH₃COOH) reacts with the added OH⁻ ions to form acetate ions (CH₃COO⁻) and water (H₂O), thereby minimizing the increase in pH.
Equilibrium in Buffers
The buffer maintains an equilibrium between the weak acid and its conjugate base (or the weak base and its conjugate acid). This equilibrium allows the buffer to absorb added H⁺ or OH⁻ ions without significantly altering the pH.
Properties of Buffers
1. Buffer Capacity
Definition: Buffer capacity is the measure of a buffer solution’s ability to resist pH change upon the addition of an acid or a base.
- High Buffer Capacity: Buffers with high concentrations of the buffering components (weak acid and its conjugate base) can neutralize larger amounts of added acid or base. This means they can absorb more H⁺ or OH⁻ ions without a significant change in pH.
- Low Buffer Capacity: Buffers with low concentrations of the buffering components are quickly overwhelmed by added acids or bases, leading to larger changes in pH.
Factors Affecting Buffer Capacity:
- Concentration of Buffer Components: The higher the concentration of the weak acid and its conjugate base, the greater the buffer capacity.
- Ratio of Acid to Base: The buffer capacity is maximized when the ratio of the concentrations of the acid and base is close to 1:1. This ensures that there are sufficient amounts of both components to react with added H⁺ or OH⁻ ions.
2. Buffer Range
Definition: Buffer range is the pH range over which a buffer can effectively neutralize added acids and bases without a significant change in pH.
- Effective Buffer Range: Typically, a buffer is most effective within ±1 pH unit of the pKa of the weak acid. This is because within this range, the concentrations of the weak acid and its conjugate base are sufficient to neutralize added H⁺ or OH⁻ ions.
Factors Affecting Buffer Range:
- pKa of the Weak Acid: The buffer range is centered around the pKa value of the weak acid in the buffer system. The effective buffering range is usually considered to be from pKa – 1 to pKa + 1.
3. pH of Buffer Solutions
The pH of a buffer solution can be calculated using the Henderson-Hasselbalch equation:
![]()
- [A⁻]: Concentration of the conjugate base.
- [HA]: Concentration of the weak acid.
Significance:
- This equation allows for the calculation of the pH of a buffer solution based on the known concentrations of the acid and its conjugate base.
- It shows that the pH of a buffer solution depends on the ratio of the concentrations of the conjugate base to the weak acid, rather than their absolute concentrations.
4. Buffer Effectiveness
Definition: Buffer effectiveness refers to the ability of a buffer solution to maintain a stable pH when acids or bases are added.
- Resilience to pH Changes: A buffer is considered effective if it can resist changes in pH when small amounts of acid or base are added. This effectiveness is a combination of the buffer capacity and the buffer range.
Significance:
- The effectiveness of a buffer is important in applications where pH stability is crucial, such as in biochemical reactions and industrial processes.
5. Biological Significance
Definition: Buffers are vital in biological systems to maintain the pH within narrow limits necessary for proper biochemical functioning.
Examples:
- Blood Buffer System: The bicarbonate buffer system (H₂CO₃/HCO₃⁻) maintains the pH of blood around 7.4, which is essential for proper physiological function.
- Cellular Buffers: Phosphate buffers (H₂PO₄⁻/HPO₄²⁻) help maintain the pH within cells, which is crucial for enzyme activity and metabolic processes.
Steps to Prepare a Buffer Solution
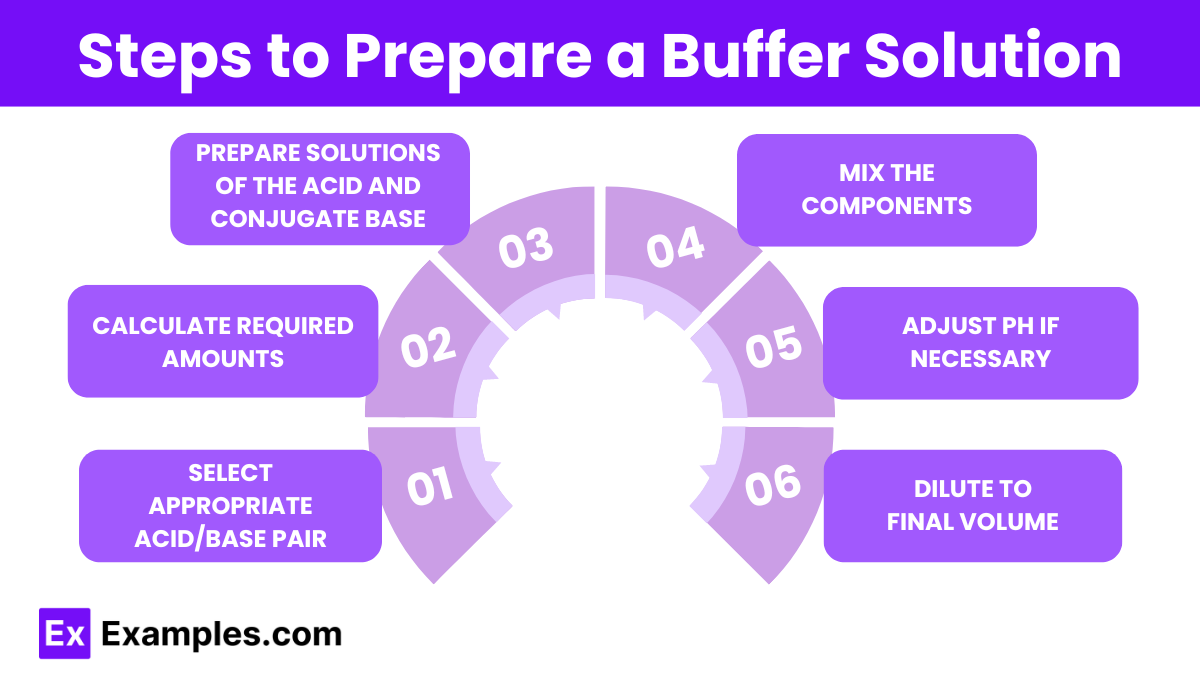
- Select Appropriate Acid/Base Pair:
- Choose a weak acid and its conjugate base or a weak base and its conjugate acid based on the desired pH and buffer range.
- Ensure the pKa of the chosen weak acid is close to the desired pH (within ±1 pH unit).
- Calculate Required Amounts:
- Use the Henderson-Hasselbalch equation to determine the ratio of the concentrations of the acid and conjugate base needed to achieve the desired pH.
- Henderson-Hasselbalch equation:
![Rendered by QuickLaTeX.com \text{pH} = \text{pKa} + \log \left( \frac{[\text{A⁻}]}{[\text{HA}]} \right)](https://www.examples.com/wp-content/ql-cache/quicklatex.com-64e3fed854cf79e6ba0139fbe0058cbc_l3.png)
- [A⁻]: Concentration of the conjugate base.
- [HA]: Concentration of the weak acid.
- Prepare Solutions of the Acid and Conjugate Base:
- Dissolve the calculated amounts of the weak acid (HA) and its conjugate base (A⁻) in distilled water.
- Mix the Components:
- Combine the solutions of the weak acid and conjugate base in the correct proportions to achieve the desired buffer composition.
- Adjust pH if Necessary:
- Use a pH meter to measure the pH of the buffer solution.
- If the pH is not within the desired range, adjust it by adding small amounts of a strong acid (e.g., HCl) or a strong base (e.g., NaOH).
- Add the acid or base dropwise and stir the solution continuously until the desired pH is reached.
- Dilute to Final Volume:
- After adjusting the pH, dilute the buffer solution to the final desired volume with distilled water.
- Ensure thorough mixing to achieve a uniform buffer solution.
Examples of Common Buffers
- Phosphate Buffer: Consists of dihydrogen phosphate (H₂PO₄⁻) as the weak acid and hydrogen phosphate (HPO₄²⁻) as the conjugate base. It has a pH range of approximately 5.8 to 8.0.
- Carbonate Buffer: Comprises carbonic acid (H₂CO₃) as the weak acid and bicarbonate (HCO₃⁻) as the conjugate base. Its pH range is approximately 6.1 to 8.4.
- Tris Buffer: Includes tris (tris(hydroxymethyl)aminomethane) as the weak base and its conjugate acid, Tris-H⁺. The pH range is approximately 7.0 to 9.0.
- Acetate Buffer: Contains acetic acid (CH₃COOH) as the weak acid and acetate (CH₃COO⁻) as the conjugate base. It has a pH range of approximately 3.7 to 5.6.
- Citrate Buffer: Composed of citric acid (H₃C₆H₅O₇) as the weak acid and citrate (C₆H₅O₇³⁻) as the conjugate base. The pH range is approximately 3.0 to 6.2.