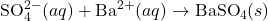Learning Objectives
By the end of this lesson, students will be able to identify and write balanced chemical equations, differentiate between different types of reactions, and interpret molecular models and reaction mechanisms to predict the outcomes of chemical processes. Students will also learn to write word equations, convert them into skeleton and balanced chemical equations, and understand the significance of ionic and net ionic equations. Additionally, students will gain the skills to classify reactions into synthesis, decomposition, single replacement, double replacement, and combustion reactions, and use molecular models to visualize chemical changes at the molecular level.
Introduction
Understanding how to represent chemical reactions is fundamental in AP Chemistry. These representations, including word equations, skeleton equations, balanced equations, ionic equations, and net ionic equations, provide a structured way to describe how reactants transform into products. Additionally, recognizing different reaction types and utilizing molecular models and reaction mechanisms helps in visualizing and predicting the outcomes of chemical processes.
Types of Chemical Equations
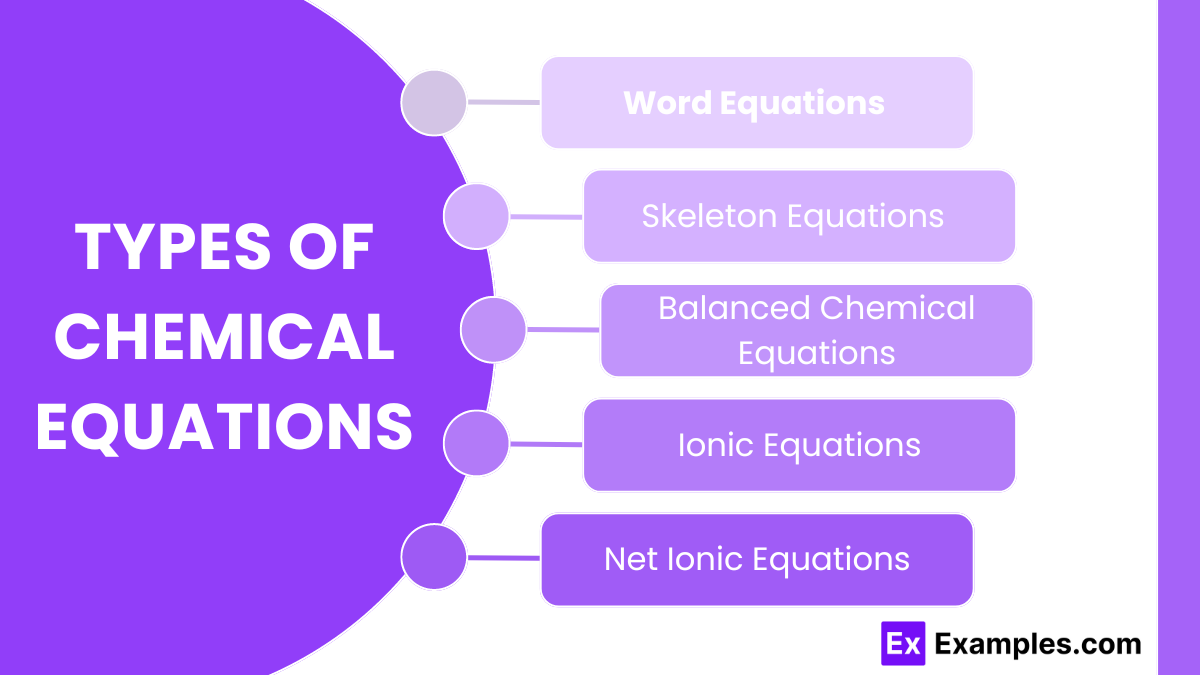
1. Word Equations
Word equations describe the reactants and products of a chemical reaction using their names. They are the simplest form of chemical equations and provide a straightforward description of the substances involved. Example: ![]()
In this reaction, methane and oxygen react to form carbon dioxide and water.
2. Skeleton Equations
Skeleton equations use chemical formulas to represent the reactants and products but do not indicate the relative amounts of each. This form is an intermediate step before balancing the equation.
Example: ![]()
Here, CH₄ represents methane, O₂ represents oxygen, CO₂ represents carbon dioxide, and H₂O represents water.
3. Balanced Chemical Equations
Balanced chemical equations ensure that the number of atoms for each element is the same on both sides of the equation, reflecting the Law of Conservation of Mass. This step is essential for accurately representing the reaction.
Example: ![]()
In this balanced equation, there are 4 hydrogen atoms and 2 oxygen atoms on both sides, ensuring mass conservation.
4. Ionic Equations
Ionic equations show the species that are actually present in the reaction mixture, including dissociated ions. This form is particularly useful for reactions occurring in aqueous solutions where ionic compounds dissociate into their constituent ions.
Example: ![]()
![]()
This equation shows sodium chloride and silver nitrate dissociating into their ions in aqueous solution, forming solid silver chloride and aqueous sodium nitrate.
5. Net Ionic Equations
Net ionic equations show only the species that undergo a change during the reaction, omitting spectator ions that do not participate in the actual chemical change. This provides a clearer picture of the essential chemical changes.
Example: ![]()
In this reaction, only the chloride ions and silver ions react to form solid silver chloride, highlighting the actual chemical change taking place.
Types of Chemical Reactions
- Synthesis: Two or more substances combine to form a new compound. A + B → AB
- Decomposition: A single compound breaks down into two or more simpler substances. AB → A + B
- Single Replacement: One element replaces another in a compound. A + BC → AC + B
- Double Replacement: Exchange of ions between two compounds. AB + CD → AD + CB
- Combustion: A substance combines with oxygen, releasing energy. CₓHᵧ + O₂ → CO₂ + H₂O
Molecular Models
Molecular models are visual representations that help in understanding the structure, geometry, and interactions of molecules. They are essential tools in chemistry for visualizing how atoms are arranged and how they bond together to form molecules.
1. Ball-and-Stick Models
Ball-and-stick models use spheres to represent atoms and rods or sticks to represent chemical bonds. The spheres are typically color-coded according to the element they represent (e.g., white for hydrogen, black for carbon, red for oxygen).
Advantages
- Clear Visualization of Bond Angles and Lengths: These models help in understanding the geometric arrangement of atoms in a molecule.
- Identifying Molecular Geometry: They are useful for studying the shapes of molecules (e.g., linear, trigonal planar, tetrahedral).
2. Space-Filling Models
Space-filling models (also known as CPK models) represent atoms as solid spheres that are proportionally sized to their van der Waals radii. These spheres are colored according to the element and overlap to show the bonds between atoms.
Advantages
- Realistic Representation of Molecular Size and Shape: These models provide a more accurate depiction of how molecules occupy space.
- Understanding Molecular Interactions: They are useful for studying how molecules fit together in reactions and biological processes.
3. Structural Formulas
Structural formulas are 2D representations that show the arrangement of atoms in a molecule and how they are bonded. Lines represent bonds between atoms.
Advantages
- Simplified View: Easier to draw and interpret compared to 3D models.
- Highlighting Functional Groups: Useful for identifying specific groups of atoms within a molecule.
4. Lewis Dot Structures
Lewis dot structures represent the valence electrons of atoms within a molecule. Dots are used to show lone pairs of electrons, and lines represent bonding pairs.
Advantages
- Valence Electron Visualization: Helps in understanding the electron distribution in a molecule.
- Predicting Molecular Shape and Reactivity: Useful for determining the formal charge and resonance structures.
5. Three-Dimensional Molecular Models
Three-dimensional molecular models can be physical or digital representations that show the spatial arrangement of atoms in a molecule.
Advantages
- Interactive and Dynamic: Digital 3D models can be rotated and manipulated to view the molecule from different angles.
- Detailed Visualization: Useful for complex molecules and large biomolecules like proteins and nucleic acids.
Reaction Mechanisms
Reaction mechanisms provide a detailed, step-by-step description of how a chemical reaction occurs at the molecular level.
Key Concepts in Reaction Mechanisms
1. Elementary Steps
Elementary steps are the simplest individual events that occur during a reaction. Each step involves a small number of molecules and represents a single stage in the overall mechanism.
2. Reaction Intermediates
Reaction intermediates are species that are formed in one elementary step and consumed in a subsequent step. They do not appear in the overall balanced equation for the reaction.
3. Rate-Determining Step
The rate-determining step is the slowest step in a reaction mechanism. It controls the overall rate of the reaction because the entire process cannot proceed faster than this step.
4. Catalysts
Catalysts are substances that increase the rate of a reaction without being consumed in the process. They provide an alternative pathway with a lower activation energy.
5. Transition States
Transition states are high-energy states that occur during the transformation of reactants into products. They represent points along the reaction pathway where old bonds are breaking, and new bonds are forming.
Practice Problems
1) Balance the following chemical equations.
- Equation:
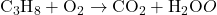
- Equation:
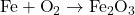
Solution:
- Balanced Equation:
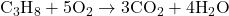
- Balanced Equation:
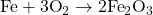
2) Write the complete ionic equation for the reaction between aqueous solutions of sodium sulfate and barium chloride.
- Reaction:
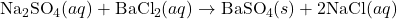
Solution:
- Complete Ionic Equation:
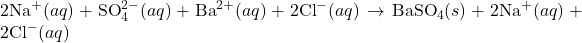
3) Write the net ionic equation for the reaction between aqueous solutions of sodium sulfate and barium chloride.
- Reaction:
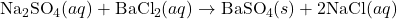
Solution:
- Net Ionic Equation: