Learning Objectives
For the AP Chemistry exam, you should aim to understand the distinct properties and behaviors of solids, liquids, and gases. Focus on mastering the characteristics of each state, including particle arrangement, density, compressibility, and kinetic energy. Learn to identify and describe different types of solids (crystalline and amorphous) and the intermolecular forces that hold them together. Comprehend the various intermolecular forces in liquids and their effects on properties like viscosity, surface tension, and vapor pressure. Familiarize yourself with the gas laws (Boyle’s, Charles’s, Avogadro’s, and the Ideal Gas Law) and the Kinetic Molecular Theory, as well as the deviations of real gases from ideal behavior. Understand phase changes, heating curves, and phase diagrams, including the significance of the triple point and critical point.
Introduction
In chemistry, understanding the fundamental properties and behaviors of solids, liquids, and gases is essential. These three states of matter differ in particle arrangement, energy, and interactions, influencing their physical properties and how they respond to changes in temperature and pressure. Solids have a fixed shape and volume with closely packed particles, liquids have a definite volume but take the shape of their container due to moderately spaced particles, and gases have neither a fixed shape nor volume, with particles that move freely and rapidly.
Solids
What are Solids?
Solids are a state of matter characterized by having a definite shape and volume, with particles that are closely packed in a fixed arrangement. The particles in a solid vibrate in place but do not move freely, resulting in a rigid structure. Solids are incompressible and typically have higher densities compared to liquids and gases.
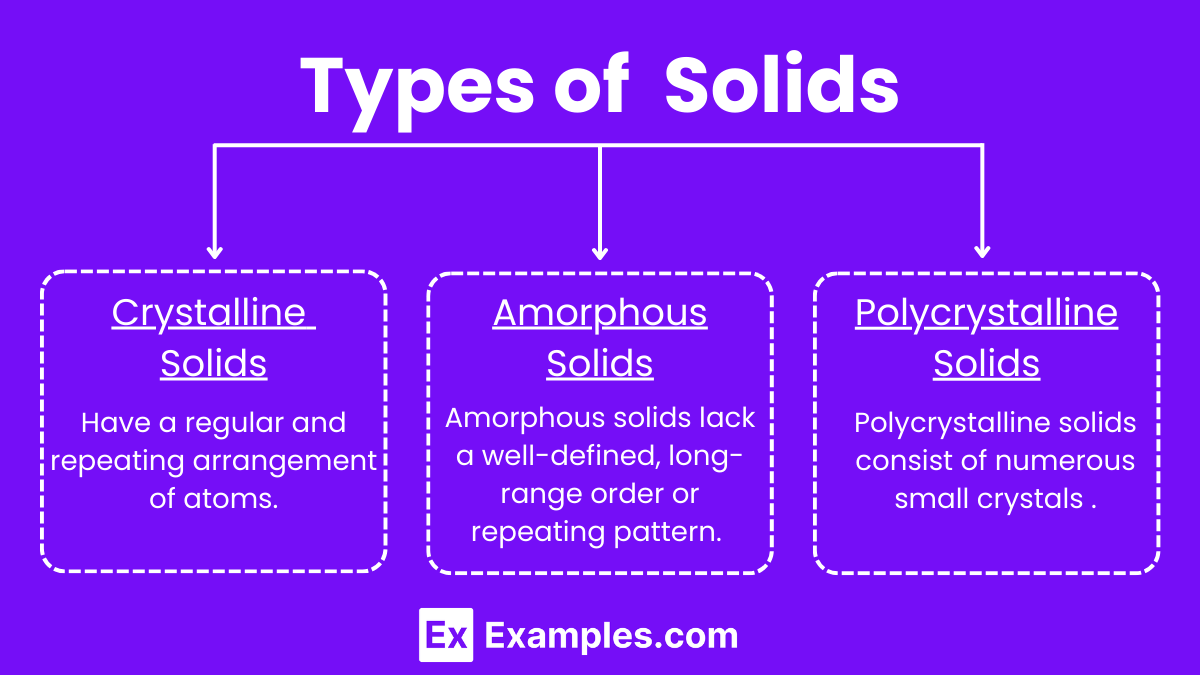
Types of Solids
Crystalline Solids
Crystalline solids have a highly ordered, repeating arrangement of atoms, ions, or molecules. This regular pattern extends throughout the material, giving it a distinct geometric shape.
Examples:
- Ionic Solids: Composed of positive and negative ions held together by electrostatic forces.
- Example: Sodium chloride (NaCl)
- Covalent Network Solids: Atoms are connected by covalent bonds in a continuous network.
- Example: Diamond (carbon)
- Molecular Solids: Molecules are held together by intermolecular forces like hydrogen bonds, dipole-dipole interactions, and London dispersion forces.
- Example: Ice (solid H₂O)
- Metallic Solids: Positive metal ions surrounded by a sea of delocalized electrons.
- Example: Iron (Fe)
Amorphous Solids
Amorphous solids lack a well-defined, long-range order or repeating pattern. Their atomic or molecular arrangement is more random, similar to that found in liquids, but they retain a solid form.
Examples:
- Glass: Made primarily of silicon dioxide (SiO₂) without a crystalline structure.
- Rubber: Polymers arranged in a disordered fashion.
- Plastic: Composed of long chains of polymers that do not have a regular pattern.
Polycrystalline Solids
Polycrystalline solids consist of numerous small crystals or grains, each with its own orientation. These grains are randomly oriented but individually exhibit crystalline properties.
Examples:
- Metals: Common structural metals like steel and copper.
- Ceramics: Materials like porcelain and pottery.
Intermolecular Forces in Solids
Intermolecular forces are the attractive forces between molecules or atoms that hold a solid together. These forces determine many of the physical properties of the solid, such as melting point, hardness, and conductivity. The strength and type of intermolecular forces vary depending on the nature of the particles in the solid. Here are the primary types of intermolecular forces found in solids:
Ionic Solids
Description: Ionic solids are held together by strong electrostatic forces between positively and negatively charged ions. These ionic bonds are very strong, resulting in solids with high melting and boiling points.
Properties:
- High melting and boiling points
- Hard and brittle
- Conductive when melted or dissolved in water
Example: Sodium chloride (NaCl)
Covalent Network Solids
Description: Atoms in covalent network solids are connected by a network of covalent bonds, forming an extensive, rigid lattice. These solids are extremely hard and have very high melting points because breaking the structure requires breaking covalent bonds.
Properties:
- Very high melting and boiling points
- Extremely hard
- Poor conductors of electricity
Example: Diamond (carbon)
Molecular Solids
Description: Molecular solids are composed of molecules held together by relatively weak intermolecular forces, such as hydrogen bonds, dipole-dipole interactions, and London dispersion forces. These solids tend to have lower melting and boiling points compared to ionic and covalent network solids.
Properties:
- Low to moderate melting and boiling points
- Soft and often volatile
- Poor conductors of electricity
Example: Ice (solid H₂O)
Metallic Solids
Description: Metallic solids consist of positive metal ions surrounded by a “sea” of delocalized electrons. The electrostatic attraction between the metal ions and the free-moving electrons holds the solid together, providing unique properties like malleability and electrical conductivity.
Properties:
- Variable melting points
- Malleable and ductile
- Good conductors of electricity and heat
Example: Iron (Fe)
Van der Waals Solids
Description: Some molecular solids, particularly those composed of non-polar molecules, are held together primarily by London dispersion forces (a type of Van der Waals force). These are the weakest intermolecular forces and result in very low melting and boiling points.
Properties:
- Very low melting and boiling points
- Soft and often gaseous or liquid at room temperature
Example: Solid carbon dioxide (dry ice, CO₂)
Properties of Crystalline Solids
| Property | Ionic Solids | Covalent Network Solids | Molecular Solids | Metallic Solids |
|---|---|---|---|---|
| Melting Point | High | Very High | Low to moderate | Variable |
| Hardness | Hard and brittle | Very hard | Generally soft | Malleable and ductile |
| Electrical Conductivity | Conductive in molten state or solution | Poor conductors | Poor conductors | Good conductors |
| Thermal Conductivity | Low to moderate | Low | Low to moderate | High |
| Solubility in Water | Generally soluble | Insoluble | Depends on polarity | Insoluble |
Characteristics of Solids
- Definite Shape and Volume: Solids maintain a fixed shape and volume regardless of the container they are in.
- Incompressibility: Solids are not easily compressed due to the minimal space between their particles.
- High Density: Solids generally have higher densities than liquids and gases because their particles are tightly packed together.
- Low Kinetic Energy: The particles in a solid vibrate in fixed positions but do not move freely, resulting in lower kinetic energy compared to liquids and gases.
- Rigidity: Solids are rigid and maintain their shape without the need for a container, due to strong intermolecular forces and fixed particle positions.
- Fixed Melting Point: Solids have a specific melting point at which they change from a solid to a liquid, determined by the strength of the forces holding the particles together.
- Anisotropy: In crystalline solids, properties such as refractive index, thermal conductivity, and electrical conductivity vary depending on the direction of measurement.
- Slow Diffusion: Diffusion in solids occurs very slowly compared to liquids and gases due to the restricted movement of atoms or molecules through the solid.
- Thermal Expansion: When heated, solids expand slightly as the increased temperature causes particles to vibrate more vigorously, increasing the distance between them.
Examples
- Gold (Au)
- Copper (Cu)
- Sulfur (S)
- Table sugar (sucrose, C₁₂H₂₂O₁₁)
- Calcium carbonate (CaCO₃)
Liquids
What are Liquids?

Liquids are a state of matter characterized by having a definite volume but no definite shape. They take the shape of their container while maintaining a consistent volume. The particles in a liquid are closely packed but can move past one another, allowing liquids to flow and conform to the shape of their surroundings. This state of matter is incompressible and has a moderate level of kinetic energy compared to solids and gases.
Intermolecular Forces in Liquids
Intermolecular forces in liquids are the attractions between molecules that determine many of their physical properties. These forces are generally stronger than those in gases but weaker than those in solids. Here are the primary types of intermolecular forces in liquids:
Hydrogen Bonding
Description: Hydrogen bonding occurs when hydrogen is bonded to a highly electronegative atom like nitrogen (N), oxygen (O), or fluorine (F). The hydrogen atom becomes highly positive and can attract the electronegative atoms of neighboring molecules.
Properties:
- Significant effect on boiling and melting points.
- Leads to higher viscosity and surface tension.
Example: Water (H₂O), where hydrogen bonds between water molecules result in high boiling and melting points.
Dipole-Dipole Interactions
Description: These forces occur between polar molecules where there is a permanent dipole. The positive end of one molecule is attracted to the negative end of another.
Properties:
- Moderate effect on boiling and melting points.
- Influence on solubility and miscibility in polar solvents.
Example: Hydrogen chloride (HCl), where the positive hydrogen end of one molecule is attracted to the negative chlorine end of another.
London Dispersion Forces (Van der Waals Forces)
Description: These are weak intermolecular forces present in all molecules, whether polar or nonpolar. They result from temporary dipoles created by the random movement of electrons.
Properties:
- More significant in larger, more polarizable molecules.
- Contribute to the boiling and melting points of nonpolar substances.
Example: Noble gases like argon (Ar) and nonpolar molecules like methane (CH₄).
Ion-Dipole Forces
Description: These forces occur between an ion and a polar molecule. The ion is attracted to the oppositely charged end of the dipole in the polar molecule.
Properties:
- Strong interaction, significant in solutions of ionic compounds in polar solvents.
- Important in dissolution processes.
Example: Sodium ions (Na⁺) in water (H₂O), where the Na⁺ is attracted to the negative oxygen end of water molecules.
Properties of Liquids
- Viscosity: Resistance to flow; higher in liquids with stronger intermolecular forces.
- Surface Tension: Energy required to increase the surface area; caused by cohesive forces among molecules.
- Vapor Pressure: Pressure exerted by a vapor in equilibrium with its liquid; increases with temperature.
Characteristics of Liquids
Liquids
- Definite Volume, Indefinite Shape: Take the shape of their container while maintaining a fixed volume.
- Moderate Compressibility: Slightly more compressible than solids but much less so than gases.
- Intermediate Density: Generally less dense than solids but denser than gases.
- Moderate Kinetic Energy: Particles move more freely than in solids but are still closely packed.
Examples
- Ethanol (C₂H₅OH)
- Mercury (Hg)
- Acetone (C₃H₆O)
- Bromine (Br₂)
- Glycerol (C₃H₈O₃)
Gases
What are Gases?

Gases are a state of matter characterized by having neither a definite shape nor a definite volume. They expand to fill the shape and volume of their container. The particles in a gas are far apart and move freely, resulting in low density and high compressibility. This state of matter exhibits high kinetic energy, allowing gas molecules to move rapidly and independently.
Gas Laws
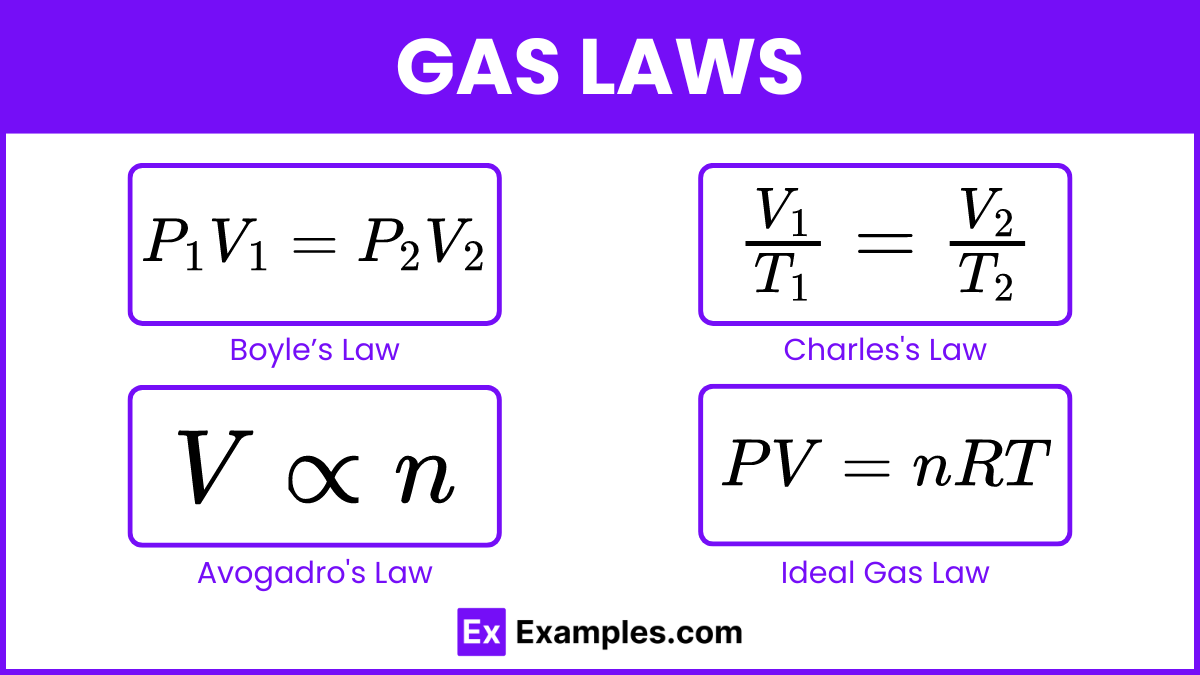
- Boyle’s Law:
(Pressure and volume are inversely proportional at constant temperature)
- Charles’s Law:
(Volume and temperature are directly proportional at constant pressure)
- Avogadro’s Law: V
n (Volume and number of moles are directly proportional at constant temperature and pressure)
- Ideal Gas Law: PV = nRT (Relates pressure, volume, number of moles, and temperature)
Kinetic Molecular Theory of Gases
- Gases consist of large numbers of particles in constant, random motion.
- The volume of gas particles is negligible compared to the container’s volume.
- There are no attractive or repulsive forces between gas particles.
- Collisions between gas particles are perfectly elastic.
- The average kinetic energy of gas particles is directly proportional to the temperature in Kelvin.
Real Gases vs. Ideal Gases
| Property/Behavior | Ideal Gases | Real Gases |
|---|---|---|
| Intermolecular Forces | No intermolecular forces between particles | Have intermolecular forces (attractive and repulsive) |
| Particle Volume | Negligible particle volume | Finite particle volume |
| Collisions | Perfectly elastic | Collisions can be non-elastic |
| Behavior at High Pressure | Obey Ideal Gas Law | Deviate significantly from Ideal Gas Law |
| Behavior at Low Temperature | Obey Ideal Gas Law | Deviate significantly from Ideal Gas Law |
| Equation of State | PV=nRT | Corrected by Van der Waals equation: |
| Condensation | Do not condense | Can condense to liquids at high pressure and low temperature |
| Example Conditions | High temperature and low pressure where interactions are minimal | Lower temperatures and higher pressures where interactions are significant |
| Compressibility Factor (Z) | Z = 1 for all conditions | Z<1 indicates attractive forces dominate, Z>1 indicates repulsive forces dominate |
Key Points
- Ideal Gases: Assumed to have no intermolecular forces and negligible particle volume. Follow the Ideal Gas Law under all conditions.
- Real Gases: Have intermolecular forces and finite particle volume. Deviate from the Ideal Gas Law at high pressures and low temperatures. These deviations are corrected by the Van der Waals equation.
Characteristics of Gases
- Indefinite Shape and Volume: Expand to fill their container.
- High Compressibility: Easily compressed due to large distances between particles.
- Low Density: Much less dense than solids and liquids.
- High Kinetic Energy: Particles move freely and rapidly.
Phase Changes
Phase changes are transitions between different states of matter (solid, liquid, gas) involving the addition or removal of energy. These processes are physical changes because they do not alter the chemical composition of the substance.
Types of Phase Changes
- Melting (Fusion): Transition from solid to liquid; requires addition of heat (endothermic).
- Freezing (Solidification): Transition from liquid to solid; requires removal of heat (exothermic).
- Vaporization (Evaporation/Boiling): Transition from liquid to gas; requires addition of heat (endothermic).
- Condensation: Transition from gas to liquid; requires removal of heat (exothermic).
- Sublimation: Transition from solid directly to gas; requires addition of heat (endothermic).
- Deposition: Transition from gas directly to solid; requires removal of heat (exothermic).
Heating and Cooling Curves
- Heating Curve: A graph showing the temperature change of a substance as heat is added. It includes plateaus where phase changes occur and temperature remains constant.
- Segments:
- Solid heating
- Melting (plateau)
- Liquid heating
- Boiling (plateau)
- Gas heating
- Segments:
- Cooling Curve: A graph showing the temperature change of a substance as heat is removed. It includes plateaus where phase changes occur and temperature remains constant.
- Segments:
- Gas cooling
- Condensation (plateau)
- Liquid cooling
- Freezing (plateau)
- Solid cooling
- Segments:
Phase Diagrams
- Phase Diagram: A graphical representation of the states of matter of a substance as a function of temperature and pressure.
- Triple Point: The unique set of conditions at which all three phases (solid, liquid, gas) coexist in equilibrium.
- Critical Point: The end point of the phase equilibrium curve between liquid and gas, beyond which the substance forms a supercritical fluid with properties of both a liquid and a gas.
- Regions: Represent the conditions under which solid, liquid, and gas phases exist.
Examples of Gases
- Oxygen (O₂)
- Nitrogen (N₂)
- Methane (CH₄)
- Helium (He)
- Carbon monoxide (CO)