Learning Objectives
For the AP Chemistry exam, you should aim to master the following key concepts on solutions and mixtures: Understand the definitions and differences between mixtures and solutions, and between homogeneous and heterogeneous mixtures. Learn to identify solvents and solutes in various solutions and grasp the factors affecting solubility, including temperature, pressure, and the nature of solute and solvent. Be proficient in calculating concentrations using different units such as molarity, molality, and percent composition. Comprehend colligative properties (vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure) and their applications. Apply Raoult’s Law to determine the vapor pressure of solutions and understand the behavior of electrolytes and nonelectrolytes. Gain practical skills in preparing and diluting solutions, and familiarize yourself with laboratory techniques like chromatography and distillation.
Introduction
In chemistry, understanding the nature of mixtures and solutions is fundamental. A mixture is a combination of two or more substances where each retains its own chemical properties and identity. Mixtures can be either homogeneous or heterogeneous. Homogeneous mixtures, known as solutions, have a uniform composition throughout, with solutes dissolved at the molecular or ionic level within the solvent. Heterogeneous mixtures, on the other hand, have a non-uniform composition where the different components can often be seen and separated physically. Solutions are involved in many chemical reactions and processes, consisting of two main components: the solute, which is the substance being dissolved, and the solvent, which is the substance doing the dissolving. The behavior of solutions, including how they form, their concentration, and their physical properties, is crucial for a wide range of applications from industrial processes to biological systems.
Solutions
What are Solutions?

A mixture where the solute particles are dissolved in the solvent, creating a uniform distribution of molecules or ions that cannot be separated by simple physical means such as filtration.
Types of Solutions
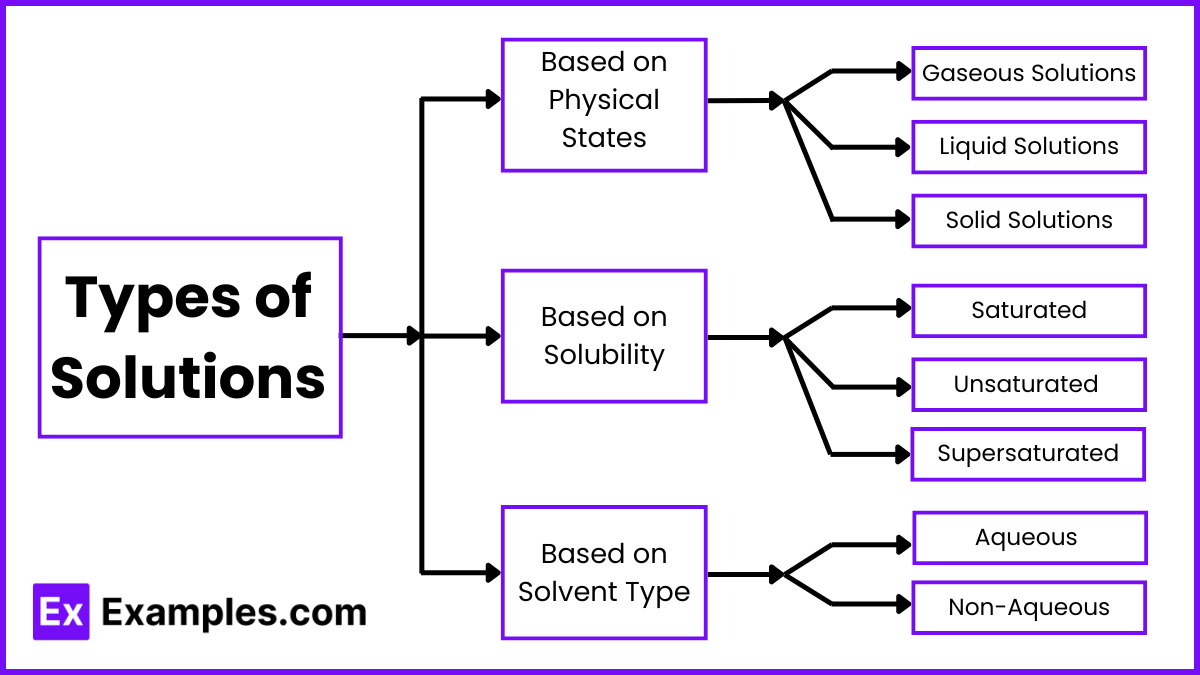
Based on Physical States
- Gaseous Solutions:
- Liquid Solutions:
- Example: Saltwater (salt dissolved in water).
- Description: The solvent is liquid, and the solute can be solid, liquid, or gas.
- Solid Solutions:
Based on Solubility
- Saturated Solutions:
- Description: Contains the maximum amount of solute that can dissolve at a given temperature. Any additional solute will not dissolve and will settle as a precipitate.
- Unsaturated Solutions:
- Description: Contains less solute than the solvent has the capacity to dissolve at a given temperature. More solute can be added and will still dissolve.
- Supersaturated Solutions:
- Description: Contains more dissolved solute than a saturated solution at the same temperature. These solutions are unstable and can precipitate the excess solute upon disturbance or seeding.
Based on Solvent Type
- Aqueous Solutions:
- Description: Solutions where water is the solvent. Common in biological and chemical processes.
- Non-Aqueous Solutions:
- Description: Solutions where the solvent is not water, such as ethanol or benzene. Used in specific chemical reactions and industrial applications.
Components of Solutions
Solvent
- Description: The component of a solution present in the largest amount. The solvent dissolves the solute and determines the phase of the solution (solid, liquid, or gas). It is the medium in which the solute particles are dispersed.
- Example: In a saltwater solution, water is the solvent.
Solute
- Description: The substance that is dissolved in the solvent. The solute is present in a smaller amount compared to the solvent. When dissolved, the solute disperses at the molecular or ionic level within the solvent.
- Example: In a saltwater solution, salt (sodium chloride) is the solute.
Characteristics
- Uniform Distribution: In a solution, the solute particles are evenly distributed throughout the solvent, resulting in a homogeneous mixture.
- Phase Change: The phase of the solute can change when it dissolves in the solvent (e.g., a solid solute dissolving in a liquid solvent).
- Interaction: The solute and solvent interact at the molecular level, and this interaction can involve physical forces (e.g., hydrogen bonding) or chemical reactions.
Types of Solutes
- Electrolytes: Solutes that dissociate into ions when dissolved in a solvent, conducting electricity. Examples include salts, acids, and bases.
- Nonelectrolytes: Solutes that do not dissociate into ions and do not conduct electricity. Examples include sugar and ethanol.
Preparation of Solutions
Steps for Preparing a Molar Solution
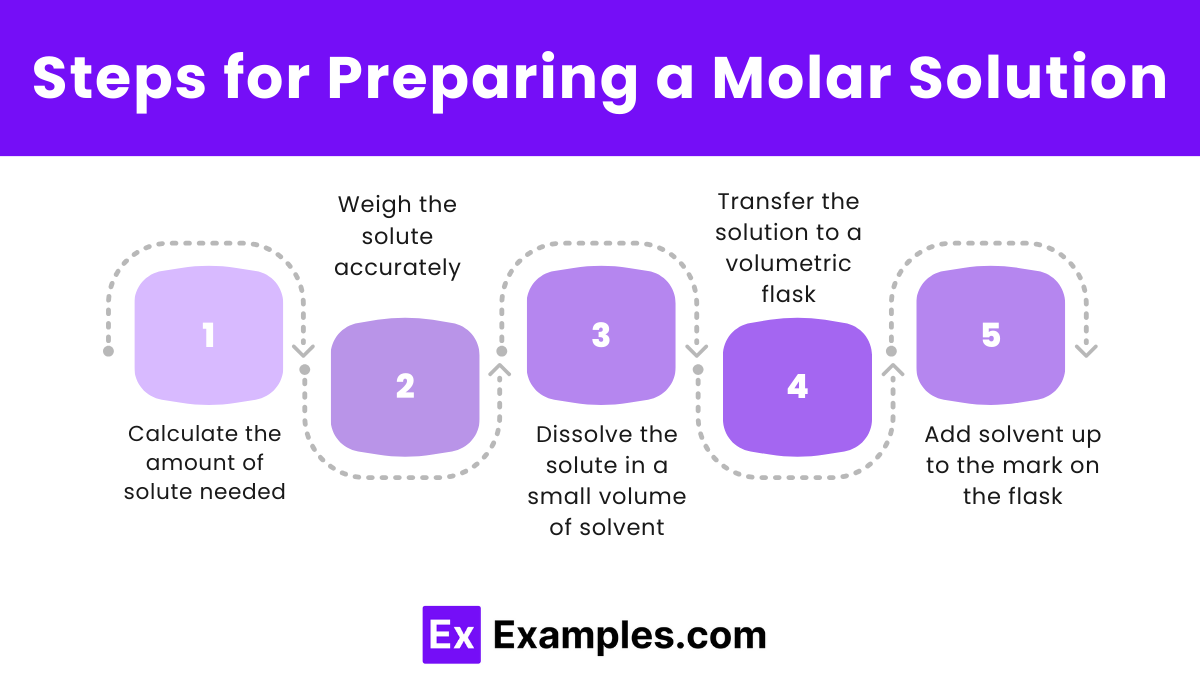
- Calculate the amount of solute needed:
- Use the formula:
- Rearrange to find the moles of solute required.
- Convert moles to grams using the molar mass of the solute.
- Use the formula:
- Weigh the solute accurately:
- Use a precise balance to measure the exact amount of solute needed.
- Dissolve the solute in a small volume of solvent:
- Pour a small portion of the solvent into a container.
- Add the solute to the solvent and stir until it is completely dissolved.
- Transfer the solution to a volumetric flask:
- Carefully transfer the dissolved solute solution into a volumetric flask.
- Add solvent up to the mark on the flask:
- Add more solvent to the flask until the solution reaches the calibration mark.
- Ensure thorough mixing by inverting the flask several times.
Steps for Diluting a Solution
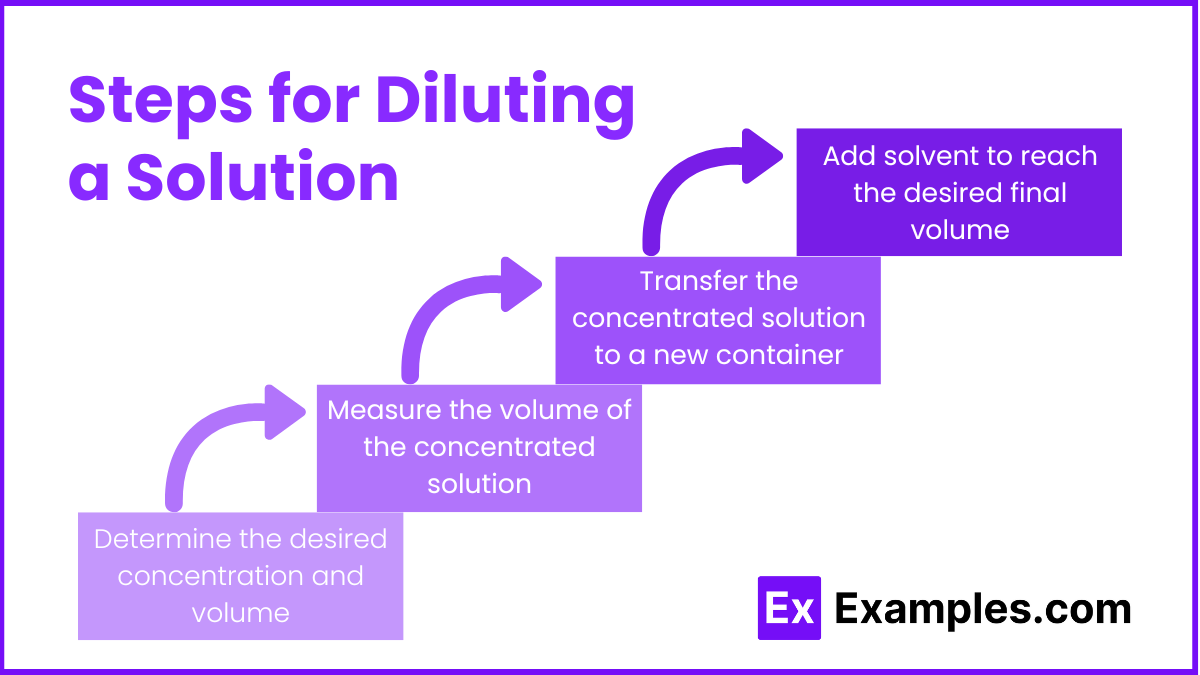
- Determine the desired concentration and volume:
- Use the dilution formula: M₁V₁ = M₂V₂
- Calculate the volume of the concentrated solution needed (V₁).
- Measure the volume of the concentrated solution:
- Use a pipette or graduated cylinder to measure the calculated volume (V₁) accurately.
- Transfer the concentrated solution to a new container:
- Pour the measured volume into a new container or volumetric flask.
- Add solvent to reach the desired final volume:
- Add solvent to the container until the solution reaches the desired final volume (V₂).
- Mix thoroughly to ensure uniform concentration.
Concentration of Solutions
Units of Concentration
- Molarity (M):
- Definition: Moles of solute per liter of solution.
- Formula:
- Example: If 2 moles of NaCl are dissolved in 1 liter of water, the molarity is 2 M.
- Molality (m):
- Definition: Moles of solute per kilogram of solvent.
- Formula:
- Example: If 1 mole of KCl is dissolved in 2 kilograms of water, the molality is 0.5 m.
- Percent Composition:
- Mass Percent:
- Definition: Mass of solute divided by the total mass of the solution, multiplied by 100.
- Formula:
- Example: If 10 grams of sugar are dissolved in 90 grams of water, the mass percent is
- Volume Percent:
- Definition: Volume of solute divided by the total volume of the solution, multiplied by 100.
- Formula:
- Example: If 20 mL of ethanol is mixed with 80 mL of water, the volume percent is
- Mass Percent:
- Parts per Million (ppm):
- Definition: Mass of solute divided by the total mass of the solution, multiplied by 1,000,000.
- Formula:
- Example: If 1 milligram of solute is dissolved in 1 kilogram of water, the concentration is 1 ppm.
- Parts per Billion (ppb):
- Definition: Mass of solute divided by the total mass of the solution, multiplied by 1,000,000,000.
- Formula:
- Example: If 1 microgram of solute is dissolved in 1 kilogram of water, the concentration is 1 ppb.
Properties of Solutions
1. Colligative Properties
Colligative properties depend on the number of solute particles in a solution, not on the nature of the solute. They include:
- Vapor Pressure Lowering:
- Description: The vapor pressure of a solvent decreases when a non-volatile solute is added.
- Explanation: Solute particles occupy space at the surface, reducing the number of solvent molecules that can escape into the vapor phase.
- Boiling Point Elevation:
- Description: The boiling point of a solution is higher than that of the pure solvent.
- Formula:
- Explanation: The presence of solute particles reduces the vapor pressure, requiring a higher temperature to reach the boiling point.
- Freezing Point Depression:
- Description: The freezing point of a solution is lower than that of the pure solvent.
- Formula:
- Explanation: Solute particles disrupt the formation of a solid lattice, lowering the temperature at which the solvent freezes.
- Osmotic Pressure:
- Description: The pressure required to stop the flow of solvent into the solution through a semipermeable membrane.
- Formula: Π = MRT
- Explanation: Solvent naturally flows into the solution to equalize solute concentration, creating osmotic pressure.
2. Electrical Conductivity
- Electrolytes: Solutes that dissociate into ions in solution, conducting electricity (e.g., NaCl in water).
- Nonelectrolytes: Solutes that do not dissociate into ions and do not conduct electricity (e.g., sugar in water).
3. Viscosity
- Description: The resistance of a solution to flow.
- Explanation: Adding solute to a solvent can increase the viscosity of the solution due to interactions between solute and solvent molecules.
4. Surface Tension
- Description: The energy required to increase the surface area of the liquid.
- Explanation: Solutions with solutes that concentrate at the surface can either increase or decrease surface tension depending on the nature of the solute.
5. Density
- Description: Mass per unit volume of a solution.
- Explanation: The addition of a solute to a solvent typically increases the density of the solution due to the mass of the solute.
6. Refractive Index
- Description: A measure of how much light is bent, or refracted, as it passes through a solution.
- Explanation: Solutions with higher concentrations of solute have higher refractive indices.
7. Color
- Description: The color of a solution can be influenced by the solute.
- Explanation: Solutes that absorb specific wavelengths of light will impart color to the solution (e.g., copper sulfate solution is blue).
Raoult’s Law
Raoult’s Law states that the vapor pressure of a solvent in a solution is directly proportional to the mole fraction of the solvent. This law is applicable to ideal solutions, where the interactions between different molecules are similar to the interactions between like molecules.
Formula
where:
= vapor pressure of the solution
= mole fraction of the solvent
= vapor pressure of the pure solvent
Explanation
- Mole Fraction: The ratio of the number of moles of the solvent to the total number of moles of all components in the solution.
- Vapor Pressure: The pressure exerted by a vapor in equilibrium with its liquid phase at a given temperature.
- Ideal Solutions: Solutions that follow Raoult’s Law closely, typically involving similar intermolecular forces between the solute and solvent.
Example
If a solution contains 0.8 mole fraction of water (solvent) and the vapor pressure of pure water at a given temperature is 25 mmHg, then the vapor pressure of the solution would be:
Factors Affecting Solubility
- Nature of the Solute and Solvent: Polar solvents dissolve polar solutes; non-polar solvents dissolve non-polar solutes.
- Temperature: Solubility of solids typically increases with temperature; solubility of gases decreases with increasing temperature.
- Pressure: Solubility of gases in liquids increases with pressure (Henry’s Law).
- Common Ion Effect: Solubility of a salt decreases in the presence of a common ion.
- pH of the Solution: Solubility of weak acids or bases can change with pH adjustments.
Mixtures
What are Mixtures?

A mixture is a combination of two or more substances in which each substance retains its own chemical identity and properties. The substances in a mixture are physically combined and can be separated by physical means.
Types of Mixtures
Homogeneous Mixtures
- Description: Homogeneous mixtures have a uniform composition throughout. The components are evenly distributed at the molecular or ionic level, making it difficult to distinguish one component from another.
- Example: Saltwater is a homogeneous mixture because the salt is completely dissolved and uniformly distributed in the water, creating a single-phase solution.
Heterogeneous Mixtures
- Description: Heterogeneous mixtures have a non-uniform composition. The components are not evenly distributed, and distinct phases or parts can often be seen and physically separated.
- Example: A salad is a heterogeneous mixture because you can see and separate the individual ingredients, such as lettuce, tomatoes, and cucumbers, which are not uniformly distributed.
Separation Techniques for Mixtures
1. Filtration
- Description: A method used to separate a solid from a liquid in a heterogeneous mixture.
- Process: The mixture is poured through a filter paper in a funnel. The liquid (filtrate) passes through, while the solid (residue) remains on the filter paper.
- Example: Separating sand from water.
2. Distillation
- Description: A technique used to separate components of a homogeneous mixture based on differences in boiling points.
- Process: The mixture is heated until one component boils and turns into vapor. The vapor is then condensed back into a liquid and collected separately.
- Example: Separating ethanol from water in an alcohol-water mixture.
3. Chromatography
- Description: A method for separating components of a mixture based on their movement through a stationary phase while being carried by a mobile phase.
- Process: The mixture is applied to a stationary phase (e.g., paper, column) and the mobile phase (e.g., solvent) moves through it. Different components move at different rates, allowing them to be separated.
- Example: Separating pigments in ink.
4. Centrifugation
- Description: A technique used to separate components of a mixture based on their densities.
- Process: The mixture is spun at high speeds in a centrifuge. The denser components move to the bottom, while the less dense components remain at the top.
- Example: Separating blood components (plasma, red blood cells).
5. Decantation
- Description: A simple method to separate liquid from solids or two immiscible liquids.
- Process: The mixture is allowed to settle and the top layer is poured off, leaving the bottom layer behind.
- Example: Separating oil from water.
6. Evaporation
- Description: A technique used to separate a dissolved solid from a liquid by evaporating the liquid.
- Process: The mixture is heated until the liquid evaporates, leaving the solid residue behind.
- Example: Recovering salt from saltwater.
7. Magnetic Separation
- Description: A method used to separate magnetic materials from non-magnetic ones.
- Process: A magnet is used to attract magnetic materials from the mixture.
- Example: Separating iron filings from sand.
8. Sublimation
- Description: A technique used to separate a substance that sublimes (changes from solid to gas without becoming liquid) from a mixture.
- Process: The mixture is heated until the sublimable component vaporizes, then the vapor is condensed back to a solid.
- Example: Separating iodine from sand.