Learning Objectives
For the AP Chemistry exam, you should be able to understand and explain the structure and properties of ionic solids. Specifically, you should learn about the crystal lattice arrangement, types of unit cells (FCC, BCC, HCP), and coordination numbers. You need to understand lattice energy and the factors that affect it, such as ion charge and size. Additionally, be familiar with the physical properties of ionic solids, including their hardness, brittleness, solubility, and electrical conductivity. Lastly, be able to provide examples of common ionic solids and describe their specific crystal structures and coordination numbers.
Free AP Chemistry Practice Test
Introduction
Ionic solids are a type of chemical compound formed by the strong electrostatic attraction between positive and negative ions. These ions arrange themselves in a highly organized, repeating pattern known as a crystal lattice. This structure gives ionic solids their distinctive properties, such as high melting and boiling points, hardness, and brittleness. Understanding the structure of ionic solids helps explain why they behave the way they do and how they interact with other substances.
What are Ionic Solids?
Ionic solids are crystalline materials composed of a lattice of positive and negative ions held together by strong electrostatic forces, known as ionic bonds. These solids form a highly organized, repeating three-dimensional structure, resulting in compounds that typically exhibit high melting and boiling points, hardness, brittleness, and the ability to conduct electricity when molten or dissolved in water.
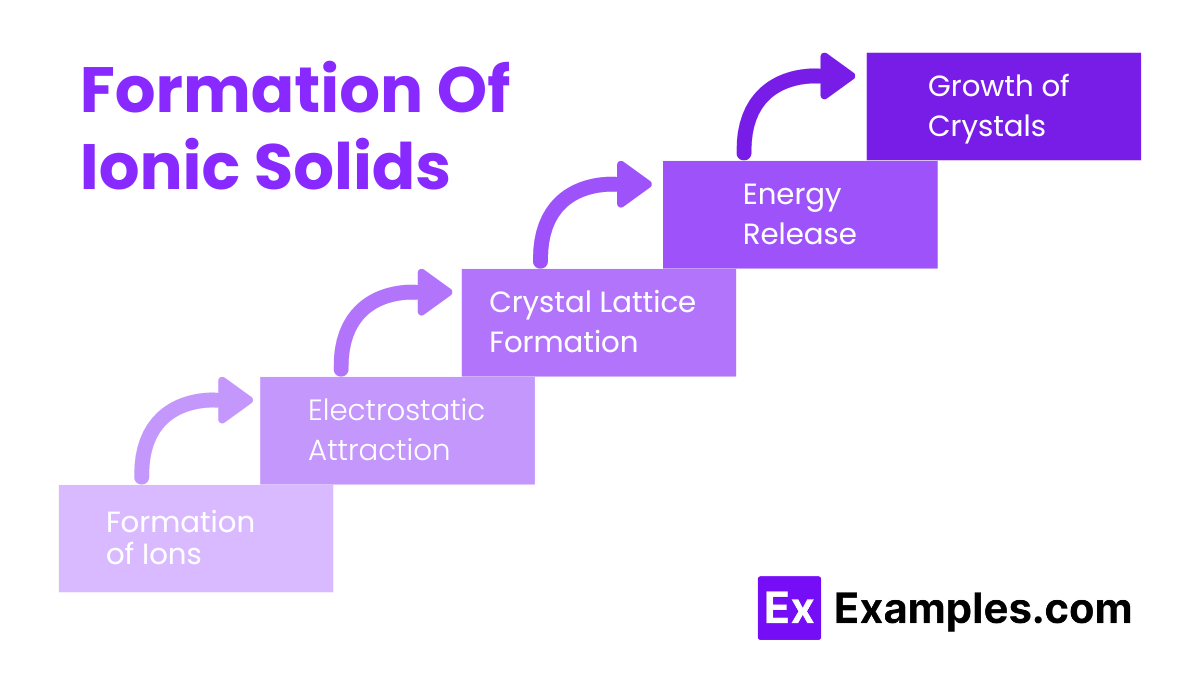
Formation Of Ionic Solids
Formation of Ions: Atoms of a metal lose electrons to form positively charged ions (cations), while atoms of a non-metal gain electrons to form negatively charged ions (anions).
Electrostatic Attraction: The oppositely charged ions attract each other due to electrostatic forces, leading to the formation of ionic bonds.
Crystal Lattice Formation: The ions arrange themselves in a regular, repeating three-dimensional pattern to minimize energy, resulting in a stable crystal lattice structure.
Energy Release: The formation of the ionic lattice releases a significant amount of energy, known as lattice energy, which stabilizes the ionic solid.
Growth of Crystals: The ionic solid grows as more ions add to the existing lattice, forming a macroscopic crystal.
Crystal Lattice Structure
The crystal lattice structure of ionic solids is a highly ordered, repeating three-dimensional arrangement of ions. This structured organization is essential to the stability and physical properties of ionic solids.
Unit Cell
The unit cell is the smallest repeating unit that reflects the symmetry and structure of the entire crystal. Each unit cell contains a specific arrangement of cations and anions.
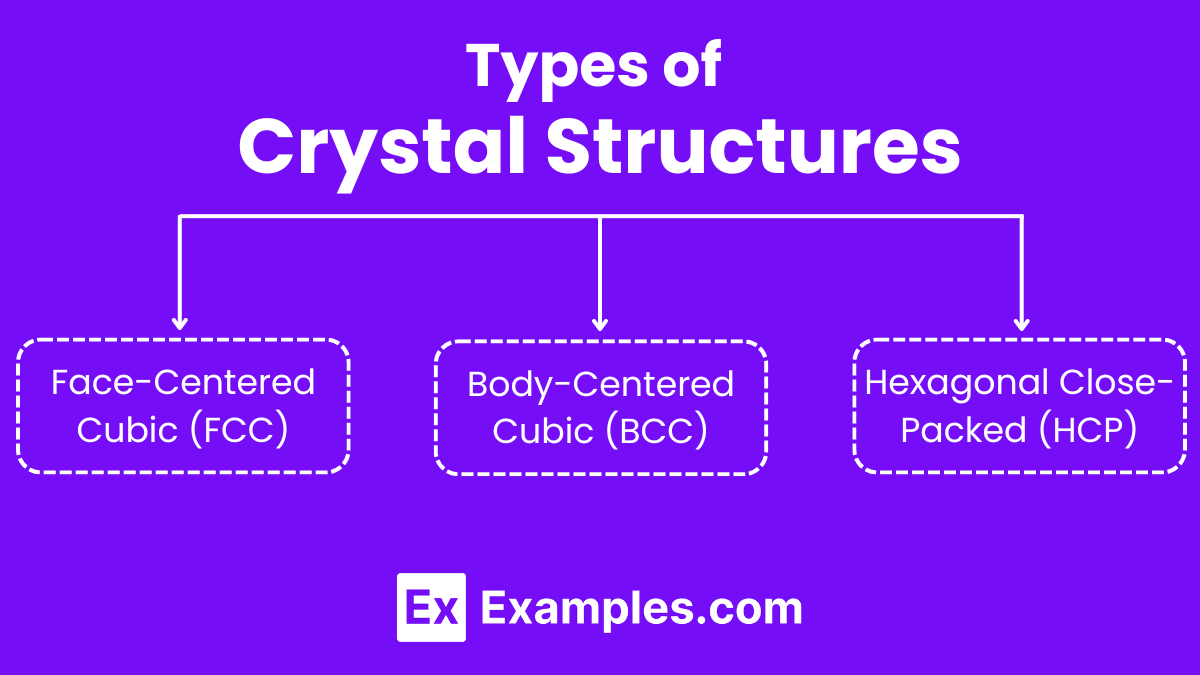
Types of Crystal Structures
Face-Centered Cubic (FCC):
In the FCC structure, each ion is surrounded by six oppositely charged ions in an octahedral arrangement.
The ions are positioned at each corner of the cube and in the center of each face of the cube.
This arrangement maximizes the distance between ions of the same charge, thereby minimizing repulsive forces.
The FCC structure is highly efficient in packing ions, leading to a stable lattice.
Example: Sodium Chloride (NaCl)
Body-Centered Cubic (BCC):
In the BCC structure, each ion is surrounded by eight oppositely charged ions in a cubic arrangement.
The ions are located at the corners of a cube with one ion at the center.
This structure allows for a slightly looser packing compared to FCC, but still maintains strong ionic bonds due to the eight neighboring ions.
BCC structures are less densely packed than FCC, but still provide stability through efficient space utilization.
Example: Cesium Chloride (CsCl)
Hexagonal Close-Packed (HCP):
In the HCP structure, each ion is surrounded by twelve oppositely charged ions.
This structure consists of layers of ions arranged in a hexagonal pattern, with each layer being offset from the one below it.
The HCP arrangement allows for maximum packing efficiency and minimizes void space within the lattice.
The HCP structure provides a high degree of stability due to the large number of nearest-neighbor interactions.
Example: Zinc Oxide (ZnO)
Coordination Number
The coordination number is the number of oppositely charged ions that surround a given ion in a crystal lattice. It helps explain the stability and properties of ionic solids.
Key Points
Definition: The number of nearest neighbor ions of opposite charge around a central ion.
Importance: Determines stability, physical properties (like hardness and melting point), and lattice energy of the ionic solid.
Common Coordination Numbers
Coordination Number | Example Compound | Description |
|---|---|---|
4 | Zinc Blende (ZnS) | Tetrahedral arrangement of ions |
6 | Sodium Chloride (NaCl) | Octahedral arrangement of ions |
8 | Cesium Chloride (CsCl) | Cubic arrangement of ions |
12 | Magnesium (Mg) | Close-packed arrangement in metals |
Lattice Energy
Lattice energy is the energy released when one mole of an ionic solid forms from its gaseous ions, reflecting the strength of the ionic bonds in the solid. High lattice energy indicates strong attractions between ions, contributing to the high melting and boiling points of ionic solids. Factors affecting lattice energy include ion charge and ion size. Higher charges on the ions result in greater lattice energy, as seen in MgO (Mg²⁺ and O²⁻) compared to NaCl (Na⁺ and Cl⁻). Similarly, smaller ions lead to greater lattice energy because the ions can get closer, increasing electrostatic attraction, like in LiCl (Li⁺ and Cl⁻) versus KCl (K⁺ and Cl⁻).
Lattice energy is crucial for understanding the properties of ionic solids, including their stability, melting and boiling points, and solubility. Compounds with high lattice energy are more stable and have higher melting and boiling points due to the strong ionic bonds. However, high lattice energy can also mean lower solubility in water, as more energy is required to separate the ions in the lattice. The Born-Haber cycle, which includes steps such as sublimation energy, bond dissociation energy, ionization energy, and electron affinity, is used to calculate lattice energy indirectly.
Factors Affecting Structure of Ionic Solids
Ion Size: Smaller ions can pack more closely together, affecting the overall arrangement and coordination number.
Ion Charge: Higher charges lead to stronger electrostatic attractions, influencing the stability and structure of the lattice.
Ratio of Cations to Anions: The stoichiometry of the compound determines the geometric arrangement of ions in the lattice.
Crystal Lattice Energy: The energy involved in forming the lattice can influence the type of crystal structure that is most stable.
Electrostatic Forces: The balance between attractive and repulsive forces among ions shapes the lattice structure.
Temperature and Pressure: These conditions can affect the formation and stability of different ionic lattice structures.
Properties and Applications
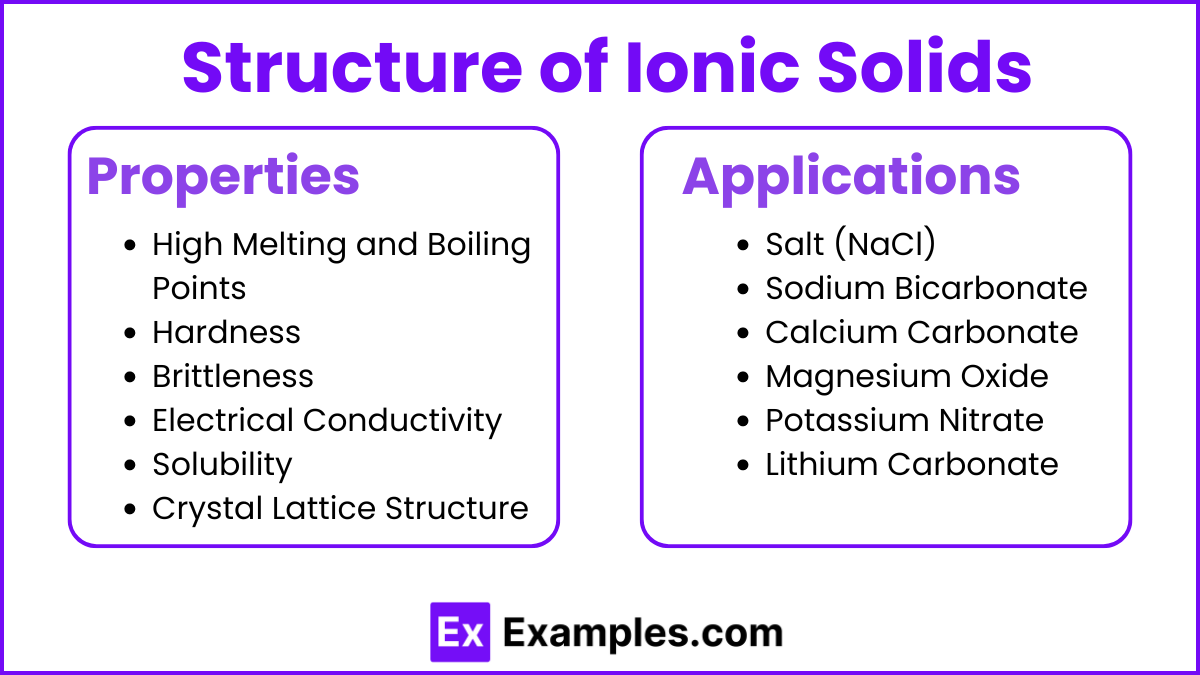
Properties
High Melting and Boiling Points: Due to strong ionic bonds.
Hardness: Ionic solids are generally hard because of the strong electrostatic forces between ions.
Brittleness: They tend to be brittle; applying stress can cause ions of like charges to align, leading to repulsion and fracture.
Electrical Conductivity: Conduct electricity when molten or dissolved in water, as the ions are free to move.
Solubility: Often soluble in polar solvents like water due to the ability of solvent molecules to overcome the electrostatic forces holding the ions together.
Crystal Lattice Structure: Highly ordered, repeating three-dimensional arrangement of ions.
Applications
Salt (NaCl): Used in food preservation and seasoning.
Sodium Bicarbonate (NaHCO₃): Used in baking as a leavening agent.
Calcium Carbonate (CaCO₃): Used in the production of cement and as a dietary calcium supplement.
Magnesium Oxide (MgO): Used in refractory materials and as an antacid.
Potassium Nitrate (KNO₃): Used in fertilizers and as a food preservative.
Lithium Carbonate (Li₂CO₃): Used in the treatment of bipolar disorder and in the manufacture of batteries.
Examples
Sodium Chloride (NaCl)
Structure: Face-Centered Cubic (FCC)
Uses: Common table salt, food preservation, de-icing roads
Magnesium Oxide (MgO)
Structure: Cubic
Uses: Refractory material in furnace linings, antacid in medicine
Calcium Fluoride (CaF₂)
Structure: Face-Centered Cubic (FCC)
Uses: Source of fluorine, production of aluminum, optics and lenses
Structure: Face-Centered Cubic (FCC)
Uses: Photography, medical imaging, sedatives
Lithium Carbonate (Li₂CO₃)
Structure: Monoclinic
Uses: Treatment of bipolar disorder, manufacturing of lithium-ion batteries