Learning Objectives
For the AP Chemistry exam, you should focus on understanding the atomic structure and bonding in metals, including metallic bonding and the arrangement of atoms in different crystal lattices (BCC, FCC, HCP). You should be able to explain the physical properties of metals such as malleability, ductility, electrical, and thermal conductivity. Additionally, learn the types of alloys (substitutional and interstitial), their structures, and properties, along with specific examples like brass, bronze, and steel. Master the interpretation of phase diagrams, including liquidus, solidus, and solvus lines, as well as eutectic and peritectic reactions. Finally, understand how alloy composition affects properties and practical applications in engineering and everyday life.
Introduction for Structure of Metals and Alloys
The structure of metals and alloys is fundamental to understanding their unique properties and applications. Metals are characterized by their metallic bonding and organized atomic arrangements in crystal lattices such as body-centered cubic (BCC), face-centered cubic (FCC), and hexagonal close-packed (HCP) structures. This orderly arrangement results in properties like high electrical and thermal conductivity, malleability, and ductility. Alloys, which are mixtures of metals with other elements, exhibit enhanced properties such as increased strength, hardness, and corrosion resistance. Studying the atomic structure, bonding, and phase behavior of metals and alloys provides insight into their wide-ranging uses in various industries and everyday applications.
Metals
What are Metals?
Metals are elements that readily form positive ions (cations) and have metallic bonds. They are characterized by their high electrical and thermal conductivity, malleability, ductility, and a lustrous appearance. Metals typically have a crystal structure in which atoms are arranged in a highly organized lattice. They are found on the left side and center of the periodic table, including groups such as the alkali metals, alkaline earth metals, transition metals, and post-transition metals.
Atomic Structure of Metals
The atomic structure of metals is defined by their arrangement of atoms and the nature of their bonding, which gives rise to their distinctive physical properties.
Metallic Bonding
- Definition: Metallic bonding is the chemical bonding that holds metal atoms together in a lattice.
- Mechanism: In metallic bonding, valence electrons are not bound to any specific atom but are free to move throughout the entire structure. This creates a “sea of electrons” that surrounds positively charged metal ions.
- Properties:
- Electrical Conductivity: The free electrons allow metals to conduct electricity efficiently.
- Thermal Conductivity: Free-moving electrons transfer thermal energy quickly through the metal.
- Malleability and Ductility: The ability of the metal ions to slide past each other without breaking the metallic bond allows metals to be hammered into sheets (malleable) and drawn into wires (ductile).
Crystal Lattices
Metals crystallize in specific geometric arrangements known as crystal lattices. The most common types are:
- Body-Centered Cubic (BCC):
- Structure: Each atom is at the center of a cube of eight other atoms.
- Atoms per Unit Cell: 2
- Examples: Iron (Fe), Chromium (Cr), Tungsten (W)
- Face-Centered Cubic (FCC):
- Structure: Atoms are located at each of the corners and the centers of all the faces of the cube.
- Atoms per Unit Cell: 4
- Examples: Aluminum (Al), Copper (Cu), Gold (Au)
- Hexagonal Close-Packed (HCP):
- Structure: Atoms are arranged in a hexagonal pattern where each atom is surrounded by 12 other atoms.
- Atoms per Unit Cell: 6
- Examples: Magnesium (Mg), Zinc (Zn), Titanium (Ti)
Coordination Number
- Definition: The coordination number is the number of nearest neighboring atoms to a given atom in the crystal lattice.
- Values:
- BCC: 8
- FCC: 12
- HCP: 12
Properties of Metals
- High Electrical Conductivity: Allows the easy flow of electric current.
- High Thermal Conductivity: Efficiently transfers heat.
- Malleability: Can be hammered or rolled into thin sheets without breaking.
- Ductility: Can be drawn into wires.
- Luster: Has a shiny, reflective surface when polished.
Example of Metallic Structures
| Metal | Crystal Structure | Coordination Number | Properties |
|---|---|---|---|
| Iron (Fe) | BCC | 8 | High strength, moderate ductility |
| Copper (Cu) | FCC | 12 | Excellent electrical conductivity, malleable |
| Magnesium (Mg) | HCP | 12 | Lightweight, good strength-to-weight ratio |
Alloys
What are Alloys?

Alloys are materials composed of two or more elements, at least one of which is a metal, that are mixed together to enhance or modify the properties of the base metal. The elements are usually melted together and then solidified to form a homogeneous mixture, which often results in improved strength, durability, corrosion resistance, or other desirable characteristics compared to the pure metal.
Types of Alloys
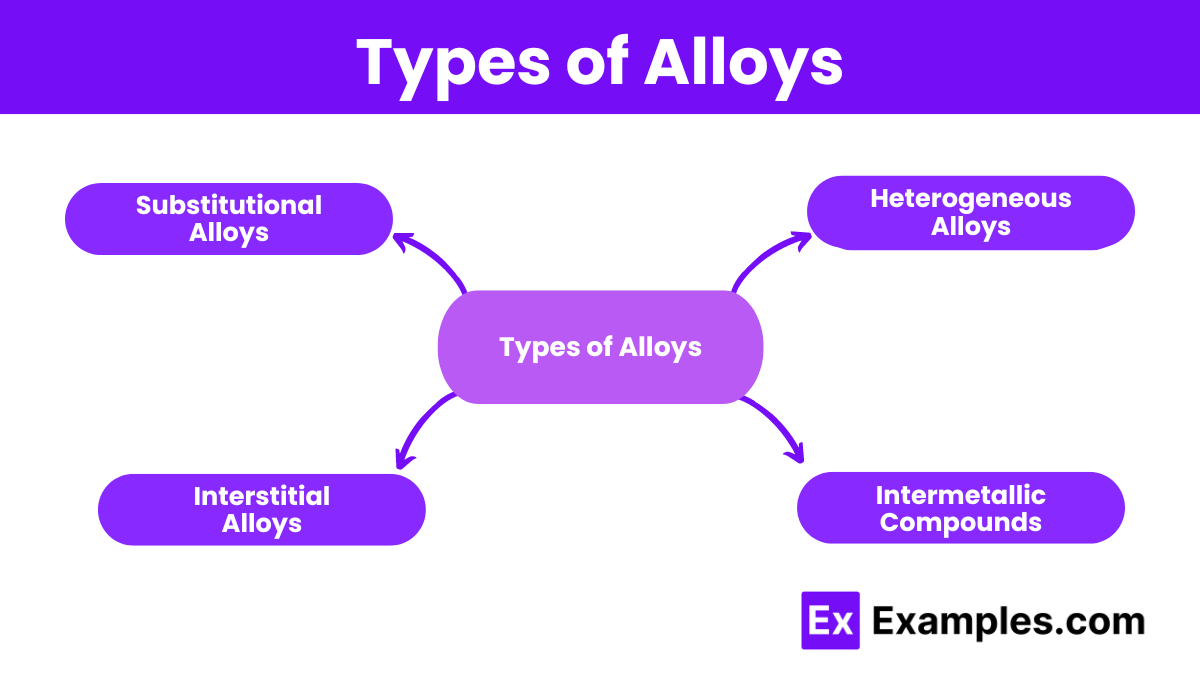
1. Substitutional Alloys
Substitutional alloys are formed when atoms of the solute metal replace atoms of the solvent metal in the crystal lattice. This type of alloy typically occurs when the constituent metals have similar atomic radii and chemical properties.
Structure: In substitutional alloys, the atoms of the solute metal take the place of some of the solvent metal atoms in the lattice. For example, in brass, zinc atoms substitute for copper atoms within the copper lattice. This substitution affects the overall properties, such as hardness and corrosion resistance.
Examples:
2. Interstitial Alloys
Interstitial alloys are formed when smaller atoms fill the interstices (spaces) between the larger atoms in a metal’s crystal lattice. This type of alloy is typically made when the solute atoms are significantly smaller than the solvent atoms.
Structure: In interstitial alloys, small atoms like carbon occupy the spaces between the larger iron atoms in the lattice of steel. The addition of these smaller atoms can significantly strengthen the metal by impeding the movement of dislocations.
Examples:
- Steel (Iron and Carbon)
- Titanium Hydride (Titanium and Hydrogen)
3. Heterogeneous Alloys
Heterogeneous alloys consist of distinct regions or phases, each with different compositions and properties. These phases can form during the solidification process when the elements involved have limited solubility in each other.
Structure: The structure of heterogeneous alloys includes different phases that are present within the material. For instance, in pearlite, alternating layers of ferrite (iron) and cementite (iron carbide) form a lamellar structure, contributing to the alloy’s strength and toughness.
Examples:
4. Intermetallic Compounds
Intermetallic compounds are alloys with a defined stoichiometric ratio and ordered crystal structure. They exhibit distinct chemical and physical properties compared to their constituent metals.
Structure: Intermetallic compounds have a highly ordered structure, often with complex arrangements of atoms. For example, nickel aluminide forms a specific cubic crystal structure where nickel and aluminum atoms occupy distinct positions, resulting in high strength and resistance to oxidation.
Examples:
- Nickel aluminide (Ni₃Al)
- Titanium aluminide (TiAl)
Factors Affecting Alloy Structure
- Elemental Composition: Types and proportions of elements in the alloy.
- Cooling Rate: Speed of cooling from the molten state.
- Heat Treatment: Processes like annealing, quenching, and tempering.
- Mechanical Working: Rolling, forging, and extrusion processes.
- Solidification Process: Method of solidifying the alloy, such as casting.
- Impurities and Additives: Presence of impurities and intentional additives.
- Phase Diagrams: Phases present at different temperatures and compositions.
- Cooling Environment: The environment in which the alloy cools (air, water, oil).
- Crystallization Patterns: Formation and growth of crystals during solidification.
Properties Influenced by Alloys Structure
- Strength: The presence of different atoms and phases can hinder dislocation movement, enhancing strength.
- Ductility: The alloy structure can either enhance or reduce ductility depending on the atomic arrangement and phase distribution.
- Hardness: Alloys can be engineered to have increased hardness through the introduction of interstitial atoms or hard phases.
- Corrosion Resistance: The alloy’s structure can influence its resistance to corrosion, with certain alloying elements providing protective properties.
- Electrical Conductivity: The presence of solute atoms can disrupt the electron flow, affecting conductivity.
Examples of Alloys
- Steel: Iron and carbon; used in construction and automotive industries.
- Brass: Copper and zinc; used in musical instruments and fittings.
- Bronze: Copper and tin; used in sculptures and bearings.
- Stainless Steel: Iron, chromium, and nickel; used in kitchenware and medical instruments.
- Duralumin: Aluminum and copper; used in aerospace and aircraft structures.