Learning Objectives
By studying VSEPR and Bond Hybridization for the AP Chemistry exam, you should be able to predict and explain the shapes of molecules based on the Valence Shell Electron Pair Repulsion (VSEPR) theory, identify and describe the different types of molecular geometries, determine the bond angles in various molecular shapes, understand the concept of hybridization and how atomic orbitals mix to form hybrid orbitals, and accurately determine the hybridization state of atoms in molecules. Mastering these concepts will enable you to analyze molecular structures, predict their properties, and understand the spatial arrangement of atoms, which is crucial for explaining chemical reactivity and behavior.
Free AP Chemistry Practice Test
Introduction
VSEPR (Valence Shell Electron Pair Repulsion) theory and bond hybridization are essential concepts in chemistry that help us understand the 3D shapes of molecules and the types of bonds they form. VSEPR theory explains how electron pairs around a central atom repel each other and arrange themselves to minimize repulsion, determining the molecule's geometry. Bond hybridization describes the mixing of atomic orbitals to create new hybrid orbitals, allowing atoms to form bonds in specific geometries. Together, these concepts provide a framework for predicting molecular shapes and understanding chemical bonding.
VSEPR
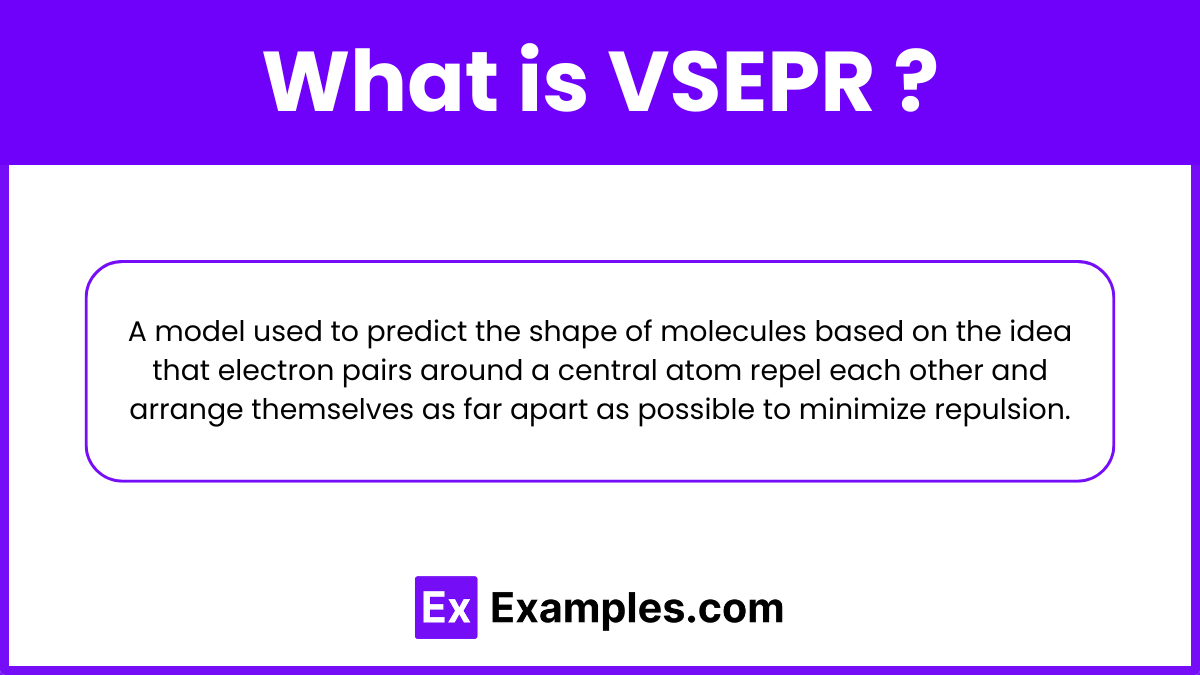
What is VSEPR?
VSEPR (Valence Shell Electron Pair Repulsion) Theory: A model used to predict the three-dimensional shape of molecules. According to VSEPR theory, electron pairs surrounding a central atom repel each other and arrange themselves as far apart as possible to minimize repulsion. This arrangement determines the molecule's geometry. VSEPR takes into account both bonding pairs (shared between atoms) and lone pairs (unshared pairs) of electrons. By considering the number of bonding and lone pairs around the central atom, VSEPR helps in predicting the overall shape and bond angles of the molecule, which are crucial for understanding its chemical properties and reactivity.
Key Principles
Electron Pair Repulsion: Electron pairs (bonding and lone pairs) around a central atom repel each other and seek to minimize repulsion by arranging themselves as far apart as possible.
Electron Domain Geometry: The overall arrangement of regions of electron density (bonding pairs and lone pairs) around the central atom determines the electron domain geometry.
Molecular Geometry: The shape of a molecule is determined by the positions of the atoms, which are influenced by the electron domain geometry. Lone pairs occupy more space than bonding pairs, affecting the final shape.
Lone Pair Effects: Lone pairs repel more strongly than bonding pairs, causing bond angles to adjust and often become smaller than the ideal angles in a perfect geometry.
Steric Number: The steric number, which is the sum of the bonding pairs and lone pairs around the central atom, helps determine the geometry:
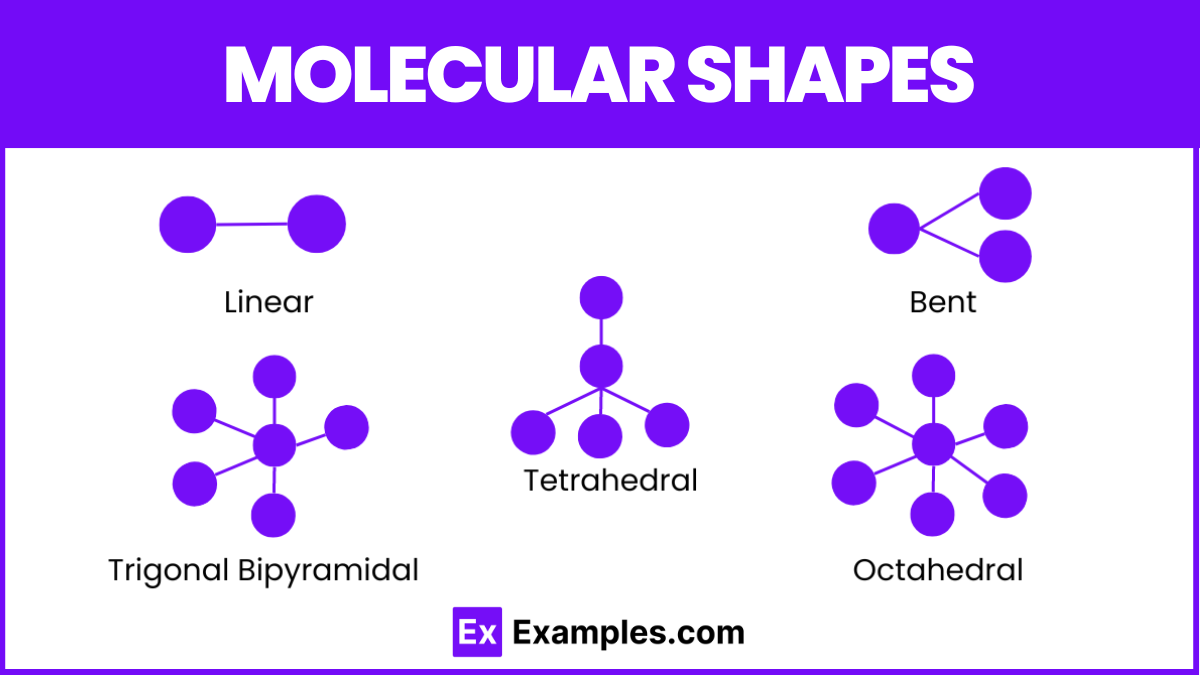
2 electron domains: Linear
3 electron domains: Trigonal Planar
4 electron domains: Tetrahedral
5 electron domains: Trigonal Bipyramidal
6 electron domains: Octahedral
Predictive Power: By using the steric number and considering the repulsion between electron pairs, VSEPR theory allows for the prediction of molecular shapes and bond angles in various molecules.
Electron Pair Geometries
Electron pair geometries describe the spatial arrangement of electron pairs (bonding and lone pairs) around a central atom. These geometries help predict the molecular shape and bond angles.
Steric Number | Electron Pair Geometry | Bond Angles (°) | Examples |
|---|---|---|---|
2 | Linear | 180 | BeCl₂, CO₂ |
3 | Trigonal Planar | 120 | BF₃, NO₃⁻ |
4 | Tetrahedral | 109.5 | CH₄, NH₄⁺ |
5 | Trigonal Bipyramidal | 90, 120 | PCl₅, SF₄ |
6 | Octahedral | 90 | SF₆, PCl₆- |
Molecular Geometries and Shapes
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. The shape of a molecule is influenced by the number of bonding pairs and lone pairs around the central atom. VSEPR theory helps predict these shapes based on the electron pair repulsions.
Linear: 2 bonding pairs, 0 lone pairs (e.g., CO2)
Trigonal Planar: 3 bonding pairs, 0 lone pairs (e.g., BF3)
Tetrahedral: 4 bonding pairs, 0 lone pairs (e.g., CH4)
Trigonal Bipyramidal: 5 bonding pairs, 0 lone pairs (e.g., PCl5)
Octahedral: 6 bonding pairs, 0 lone pairs (e.g., SF6)
Influence of Lone Pairs
Lone pairs of electrons exert greater repulsive forces than bonding pairs, which can alter bond angles and the overall shape of the molecule.
Effects of Lone Pairs on Molecular Geometry
Bent: 2 bonding pairs, 1 or 2 lone pairs (e.g., H2O, SO2)
Trigonal Pyramidal: 3 bonding pairs, 1 lone pair (e.g., NH3)
See-Saw: 4 bonding pairs, 1 lone pair (e.g., SF4)
T-Shaped: 3 bonding pairs, 2 lone pairs (e.g., ClF3)
Square Planar: 4 bonding pairs, 2 lone pairs (e.g., XeF4)
Examples
1. Methane (CH₄)
Central Atom: Carbon
Steric Number: 4 (4 bonding pairs, 0 lone pairs)
Electron Pair Geometry: Tetrahedral
Molecular Geometry: Tetrahedral
Bond Angles: 109.5°
Description: Methane has four hydrogen atoms symmetrically arranged around the central carbon atom, forming a tetrahedral shape.
2. Ammonia (NH₃)
Central Atom: Nitrogen
Steric Number: 4 (3 bonding pairs, 1 lone pair)
Electron Pair Geometry: Tetrahedral
Molecular Geometry: Trigonal Pyramidal
Bond Angles: <109.5° (approximately 107°)
Description: Ammonia has three hydrogen atoms and one lone pair around the central nitrogen atom. The lone pair causes the molecule to have a trigonal pyramidal shape, reducing the bond angle slightly.
3. Water (H₂O)
Central Atom: Oxygen
Steric Number: 4 (2 bonding pairs, 2 lone pairs)
Electron Pair Geometry: Tetrahedral
Molecular Geometry: Bent
Bond Angles: <109.5° (approximately 104.5°)
Description: Water has two hydrogen atoms and two lone pairs around the central oxygen atom. The lone pairs repel more strongly, creating a bent shape with a bond angle of about 104.5°.
4. Sulfur Hexafluoride (SF₆)
Central Atom: Sulfur
Steric Number: 6 (6 bonding pairs, 0 lone pairs)
Electron Pair Geometry: Octahedral
Molecular Geometry: Octahedral
Bond Angles: 90°
Description: Sulfur hexafluoride has six fluorine atoms symmetrically arranged around the central sulfur atom, forming an octahedral shape with 90° bond angles.
5. Phosphorus Pentachloride (PCl₅)
Central Atom: Phosphorus
Steric Number: 5 (5 bonding pairs, 0 lone pairs)
Electron Pair Geometry: Trigonal Bipyramidal
Molecular Geometry: Trigonal Bipyramidal
Bond Angles: 90°, 120°
Description: Phosphorus pentachloride has five chlorine atoms arranged in a trigonal bipyramidal shape around the central phosphorus atom, with three atoms in the equatorial plane at 120° angles and two atoms in the axial positions at 90° angles.
Bond Hybridization
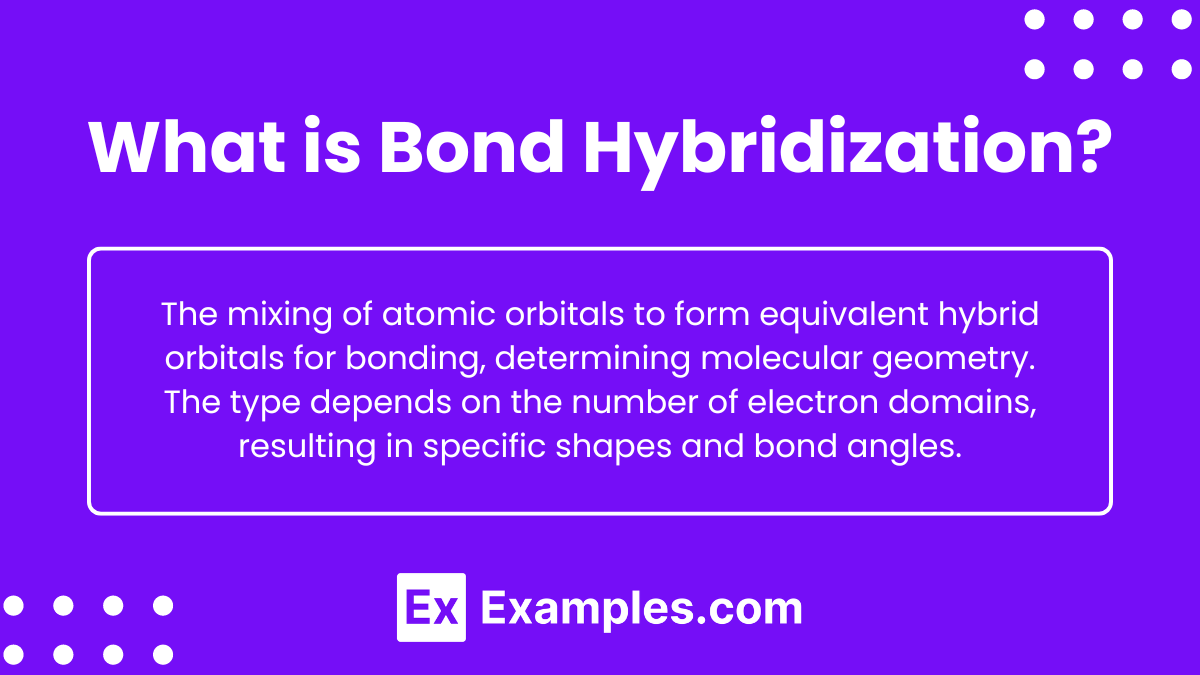
What is Bond Hybridization?
The process in which atomic orbitals mix and combine to form new, equivalent hybrid orbitals that can be used to form chemical bonds. Hybridization helps explain the observed geometries of molecules by allowing the central atom to form bonds with equal energy and appropriate spatial orientations. The type of hybridization depends on the number of electron domains (bonding pairs and lone pairs) around the central atom, and it results in specific shapes and bond angles for the molecules.
Types of Hybridization
sp Hybridization
sp hybridization involves the mixing of one s orbital and one p orbital to form two equivalent sp hybrid orbitals, each with 50% s character and 50% p character. This type of hybridization results in a linear geometry with a bond angle of 180°. An example of a molecule exhibiting sp hybridization is beryllium chloride (BeCl₂). In BeCl₂, the beryllium atom's one s orbital and one p orbital hybridize to form two sp orbitals, which align linearly to minimize electron repulsion, creating a straight-line structure.
sp² Hybridization
sp² hybridization involves the mixing of one s orbital and two p orbitals to form three equivalent sp² hybrid orbitals, each with 33.3% s character and 66.7% p character. This type of hybridization results in a trigonal planar geometry with bond angles of 120°. An example of sp² hybridization is boron trifluoride (BF₃). In BF₃, one s orbital and two p orbitals of the boron atom hybridize to form three sp² orbitals. These orbitals arrange themselves in a trigonal planar geometry to ensure minimal electron repulsion.
sp³ Hybridization
sp³ hybridization involves the mixing of one s orbital and three p orbitals to form four equivalent sp³ hybrid orbitals, each with 25% s character and 75% p character. This type of hybridization results in a tetrahedral geometry with bond angles of 109.5°. An example of sp³ hybridization is methane (CH₄). In CH₄, one s orbital and three p orbitals of the carbon atom hybridize to form four sp³ orbitals. These orbitals arrange themselves in a tetrahedral geometry to minimize repulsion between electron pairs.
sp³d Hybridization
sp³d hybridization involves the mixing of one s orbital, three p orbitals, and one d orbital to form five equivalent sp³d hybrid orbitals, each with 20% s character, 60% p character, and 20% d character. This type of hybridization results in a trigonal bipyramidal geometry with bond angles of 90° and 120°. An example of sp³d hybridization is phosphorus pentachloride (PCl₅). In PCl₅, one s orbital, three p orbitals, and one d orbital of the phosphorus atom hybridize to form five sp³d orbitals, which arrange themselves in a trigonal bipyramidal geometry.
sp³d² Hybridization
sp³d² hybridization involves the mixing of one s orbital, three p orbitals, and two d orbitals to form six equivalent sp³d² hybrid orbitals, each with approximately 16.7% s character, 50% p character, and 33.3% d character. This type of hybridization results in an octahedral geometry with bond angles of 90°. An example of sp³d² hybridization is sulfur hexafluoride (SF₆). In SF₆, one s orbital, three p orbitals, and two d orbitals of the sulfur atom hybridize to form six sp³d² orbitals. These orbitals arrange themselves in an octahedral geometry to minimize repulsion.
Characteristics of Hybrid Orbitals
sp Hybridization: Linear, 180° bond angles, two hybrid orbitals.
sp2 Hybridization: Trigonal planar, 120° bond angles, three hybrid orbitals.
sp3 Hybridization: Tetrahedral, 109.5° bond angles, four hybrid orbitals.
sp3d Hybridization: Trigonal bipyramidal, 90° and 120° bond angles, five hybrid orbitals.
sp3d2 Hybridization: Octahedral, 90° bond angles, six hybrid orbitals.
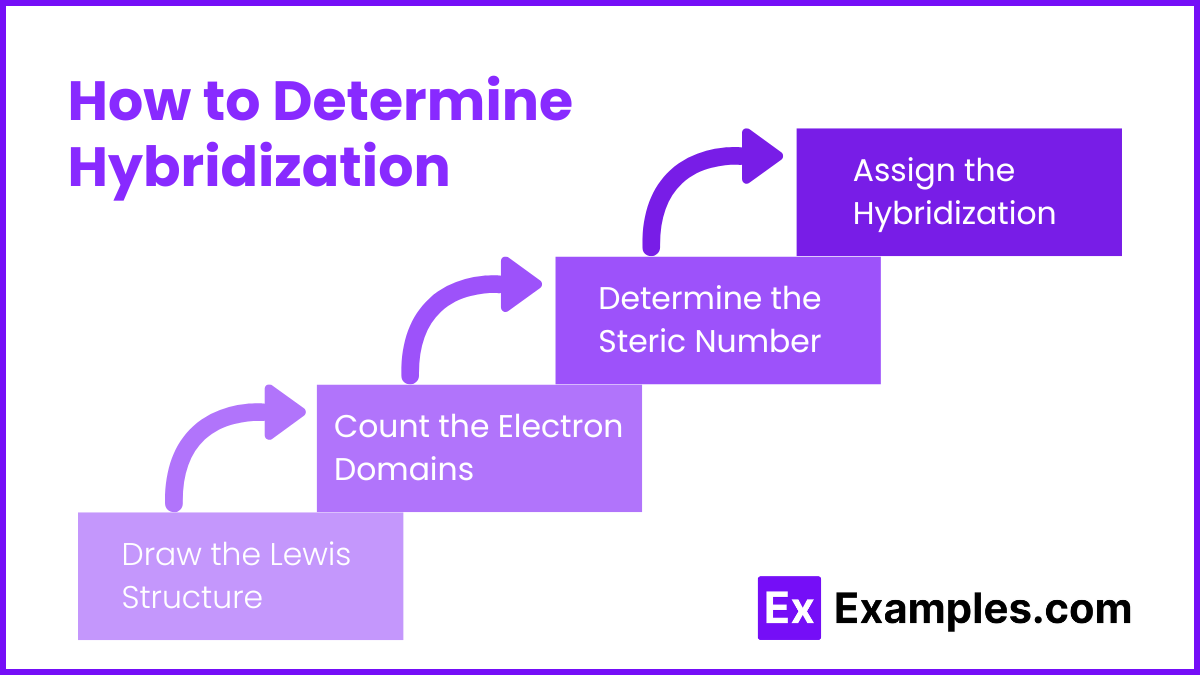
How to Determine Hybridization
Draw the Lewis Structure: Write the Lewis structure of the molecule to identify the central atom and visualize the bonding.
Count the Electron Domains: Determine the number of regions of electron density (electron domains) around the central atom. This includes:
Bonding pairs (single, double, or triple bonds count as one region each)
Lone pairs of electrons
Determine the Steric Number: The steric number is the total number of electron domains around the central atom. This will help identify the type of hybridization.
Assign the Hybridization:
2 Electron Domains: sp hybridization
Example: BeCl2 (Linear geometry, 180° bond angles)
3 Electron Domains: sp2 hybridization
Example: BF3 (Trigonal planar geometry, 120° bond angles)
4 Electron Domains: sp3 hybridization
Example: CH4 (Tetrahedral geometry, 109.5° bond angles)
5 Electron Domains: sp3d hybridization
Example: PCl5 (Trigonal bipyramidal geometry, 90° and 120° bond angles)
6 Electron Domains: sp3d2 hybridization
Example: SF6 (Octahedral geometry, 90° bond angles)
Examples
Table
Molecule | Hybridization | Bond Angles | Geometry |
|---|---|---|---|
Methane (CH₄) | sp³ | 109.5° | Tetrahedral |
sp² | 120° | Planar | |
Acetylene (C₂H₂) | sp | 180° | Linear |
Ammonia (NH₃) | sp³ | ~107° | Trigonal Pyramidal |
Sulfur Hexafluoride (SF₆) | sp³d² | 90° | Octahedral |