Acid rain, a critical issue in AP Environmental Science, significantly impacts our ecology, ecosystem, and biodiversity by introducing harmful acids into the biosphere. Formed from sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) reacting with water vapor, acid rain lowers the pH of soil and water bodies, damaging vegetation, aquatic life, and infrastructure. Understanding acid rain’s effects and control measures is essential for protecting the health and balance of our biosphere and maintaining biodiversity.
Learning Objectives
By studying acid rain, students will understand its formation and effects on organisms, including plants and animals. They will learn how acid rain contributes to climate changes and negatively impacts flora and fauna by acidifying soil and water bodies. Additionally, students will explore strategies to reduce emissions of sulfur dioxide (SO₂) and nitrogen oxides (NOₓ), mitigating damage to ecosystems. This knowledge is crucial for protecting the environment and promoting the health of diverse organisms amidst ongoing climate changes.
Causes of Acid Rain
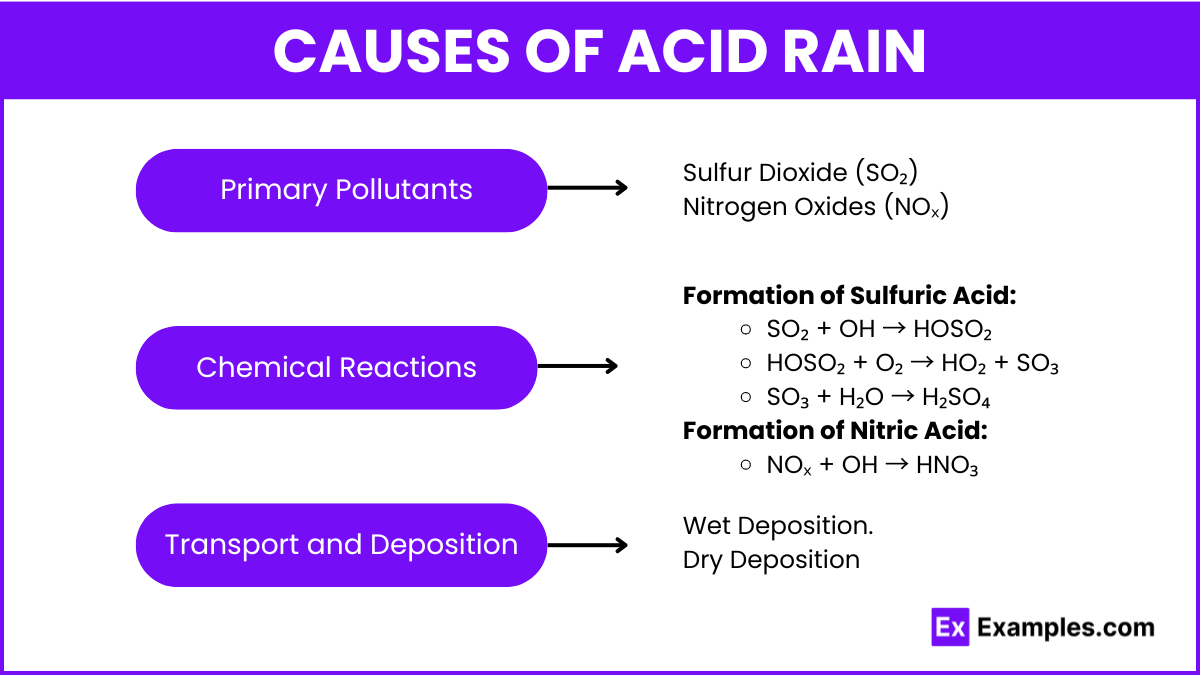
Primary Pollutants
- Sulfur Dioxide (SO₂): Emitted from burning fossil fuels, particularly coal and oil, in power plants and industrial processes.
- Nitrogen Oxides (NOₓ): Released from vehicle exhaust, industrial processes, and power generation.
Chemical Reactions
- Formation of Sulfuric Acid:
- SO₂ + OH → HOSO₂
- HOSO₂ + O₂ → HO₂ + SO₃
- SO₃ + H₂O → H₂SO₄
- Formation of Nitric Acid:
- NOₓ + OH → HNO₃
Transport and Deposition
- Pollutants can be carried long distances by wind before they react with water vapor and fall as acid rain.
- Wet Deposition: Acidic rain, snow, or fog.
- Dry Deposition: Acidic gases and particles settle out of the atmosphere in dry form.
Effects of Acid Rain
Environmental Effects
Terrestrial Ecosystems
- Soil Acidification: Lowers soil pH, leaches essential nutrients like calcium and magnesium, and releases toxic metals such as aluminum.
- Vegetation Damage: Direct damage to leaves and needles, reduced photosynthesis, and impaired growth.
- Forest Decline: Weakening of trees, making them more susceptible to disease, pests, and harsh weather.
Aquatic Ecosystems
- Water Acidification: Lowers pH of lakes, rivers, and streams.
- Fish and Aquatic Life: Harmful effects on fish and aquatic organisms, leading to reduced biodiversity. Species like trout and salmon are particularly sensitive.
- Aluminum Toxicity: Acidic water releases aluminum from soil, which can be toxic to fish and other aquatic life.
Human Health Effects
- Respiratory Issues: Inhalation of fine particles from SO₂ and NOₓ can lead to respiratory problems, including asthma and bronchitis.
- Water Quality: Acid rain can leach heavy metals into drinking water sources, posing health risks.
Material and Structural Damage
- Building Corrosion: Acid rain accelerates the deterioration of buildings and monuments, especially those made of limestone and marble.
- Infrastructure: Damage to bridges, vehicles, and other structures.
Measuring and Monitoring Acid Rain
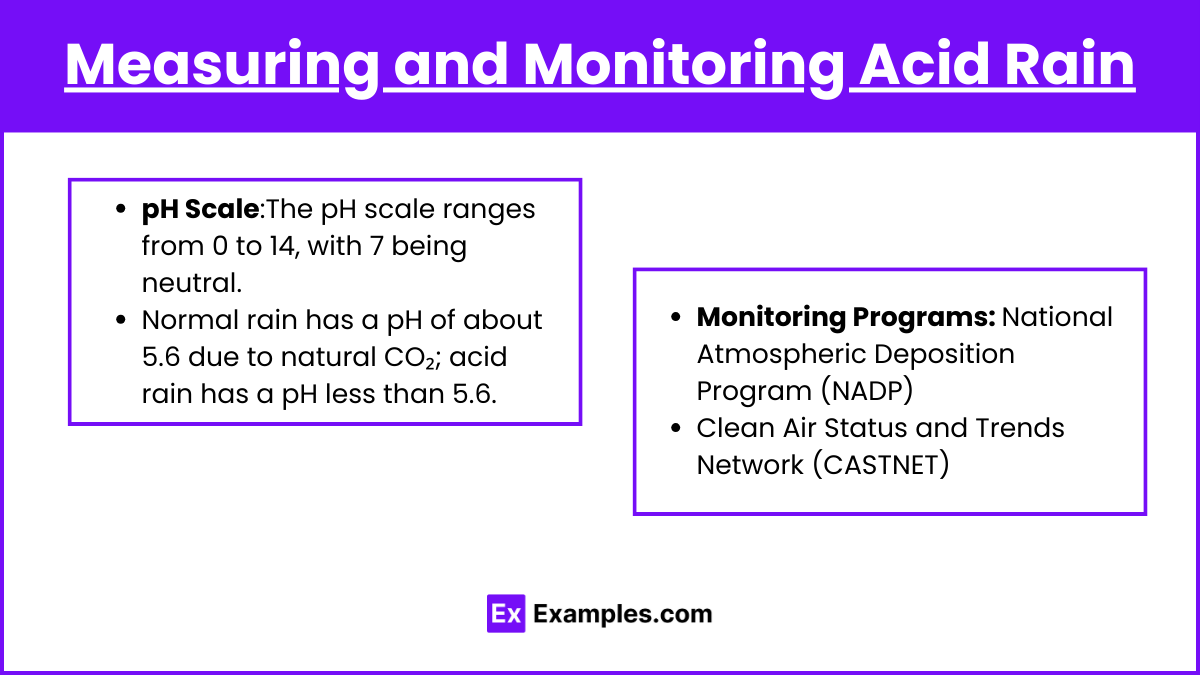
pH Scale
- The pH scale ranges from 0 to 14, with 7 being neutral.
- Normal rain has a pH of about 5.6 due to natural CO₂; acid rain has a pH less than 5.6.
Monitoring Programs
- National Atmospheric Deposition Program (NADP): Collects data on precipitation chemistry.
- Clean Air Status and Trends Network (CASTNET): Monitors dry deposition and air quality.
Control Measures for Acid Rain
Regulatory Actions
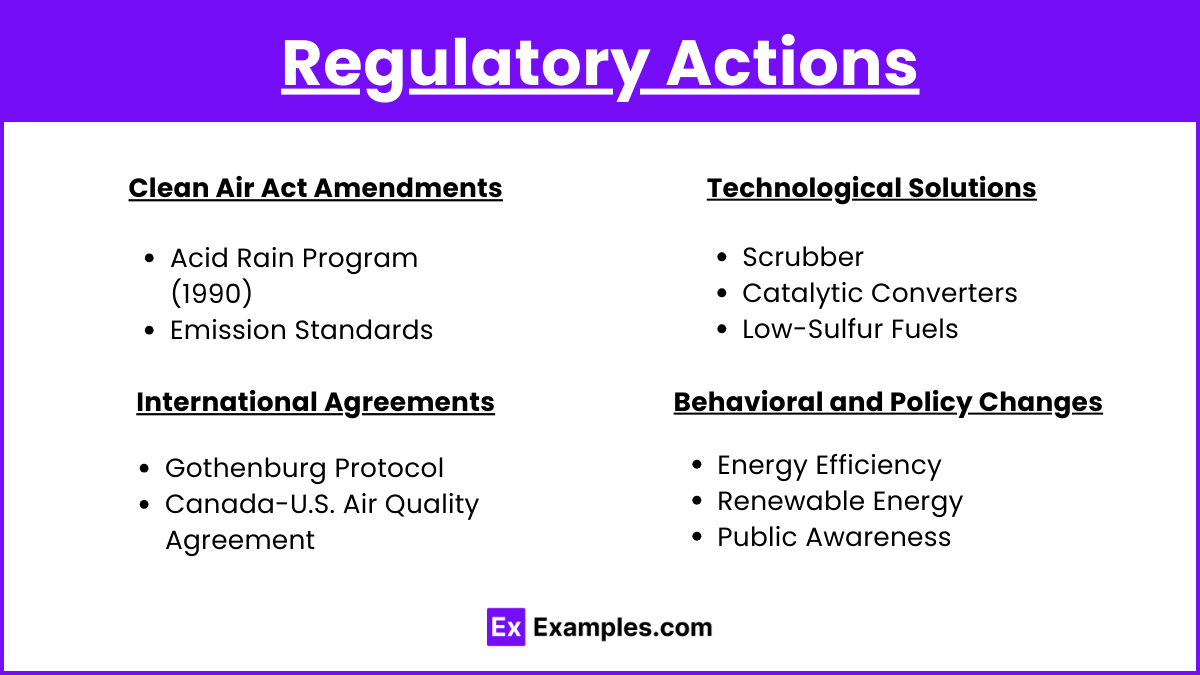
Clean Air Act Amendments
- Acid Rain Program (1990): Introduced cap-and-trade programs to reduce SO₂ and NOₓ emissions.
- Emission Standards: Set limits on emissions from power plants and industrial sources.
Technological Solutions
- Scrubbers: Devices installed in power plants to remove SO₂ from flue gases.
- Catalytic Converters: Reduce NOₓ emissions from vehicle exhaust.
- Low-Sulfur Fuels: Use of fuels with lower sulfur content.
International Agreements
- Gothenburg Protocol: Part of the UNECE Convention on Long-Range Transboundary Air Pollution (CLRTAP), aimed at reducing emissions of acidifying pollutants.
- Canada-U.S. Air Quality Agreement: Bilateral agreement to address transboundary air pollution issues, including acid rain.
Behavioral and Policy Changes
- Energy Efficiency: Reducing energy consumption through efficient appliances and practices.
- Renewable Energy: Shifting to solar, wind, and other renewable energy sources to reduce reliance on fossil fuels.
- Public Awareness: Educating the public on the causes and effects of acid rain and ways to reduce emissions.
Case Studies
Adirondack Mountains, USA
- Problem: Severe acid rain in the 1970s and 1980s led to the acidification of lakes and streams, damaging aquatic ecosystems.
- Measures: Implementation of the Clean Air Act amendments, installation of scrubbers in power plants.
- Outcome: Improved water quality and recovery of some aquatic species, though some lakes remain acidified.
Black Forest, Germany
- Problem: Acid rain caused extensive forest damage and soil acidification.
- Measures: Germany adopted strict emission controls and improved forest management practices.
- Outcome: Significant reduction in acid rain and partial recovery of forest health.


