Understanding the fundamental force of nature is crucial for mastering concepts related to interactions between particles and objects in the AP Physics exam. There are four fundamental forces: gravitational, electromagnetic, weak nuclear, and strong nuclear forces. Below are detailed notes to help you achieve a high score on your AP Physics exam.
Free AP Physics 1: Algebra-Based Practice Test
Learning Objectives
In the study of Fundamental Forces for the AP Physics exam, you will learn about the four fundamental forces of nature: gravitational, electromagnetic, strong nuclear, and weak nuclear forces. Understanding their properties, interactions, and the particles involved is crucial. You will explore how these forces govern the behavior of matter and energy, from the subatomic to the cosmic scale, and their applications in various physical phenomena and technologies. Mastering these concepts is essential for analyzing and solving complex physics problems.
The Four Fundamental Forces
1. Gravitational Force
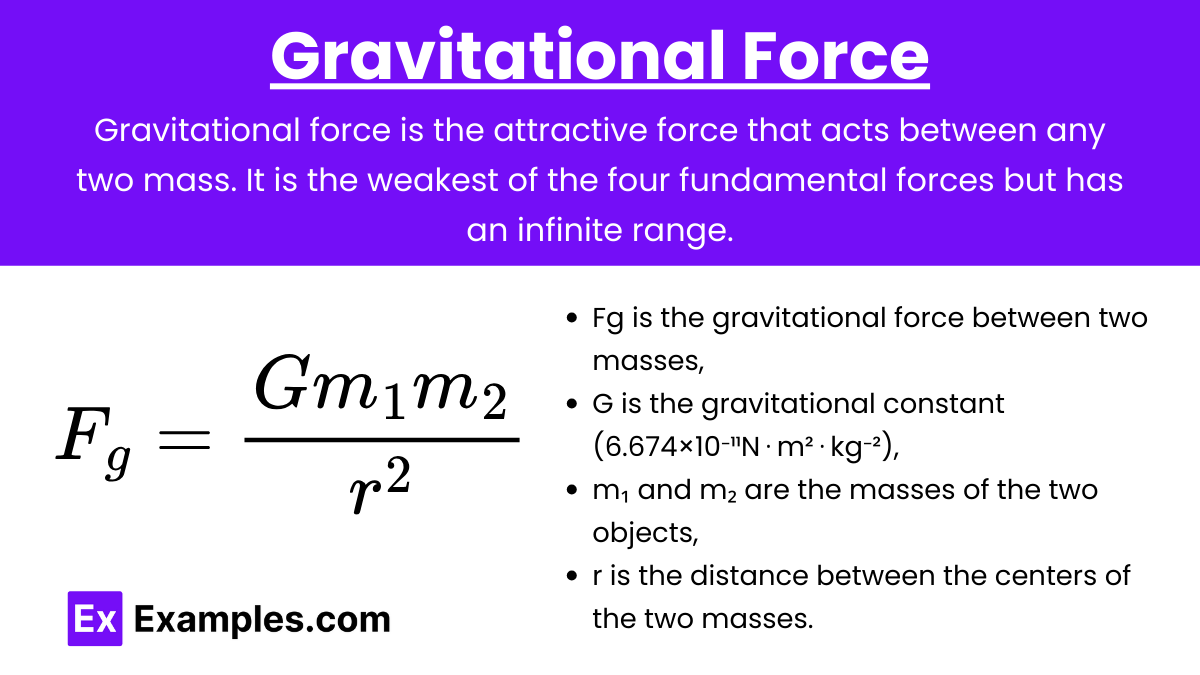
Definition: Gravitational force is the attractive force that acts between any two mass. It is the weakest of the four fundamental forces but has an infinite range.
Newton's Law of Universal Gravitation: F_g = \frac{G m_1 m_2}{r^2}
where:
Fg is the gravitational force,
G is the gravitational constant (6.674×10⁻¹¹N⋅m²⋅kg⁻²),
m₁ and m₂ are the masses,
r is the distance between the centers of the two masses.
Key Points:
Acts over large distances and governs the motion of celestial bodies.
Always attractive and acts along the line joining the centers of two masses.
2. Electromagnetic Force
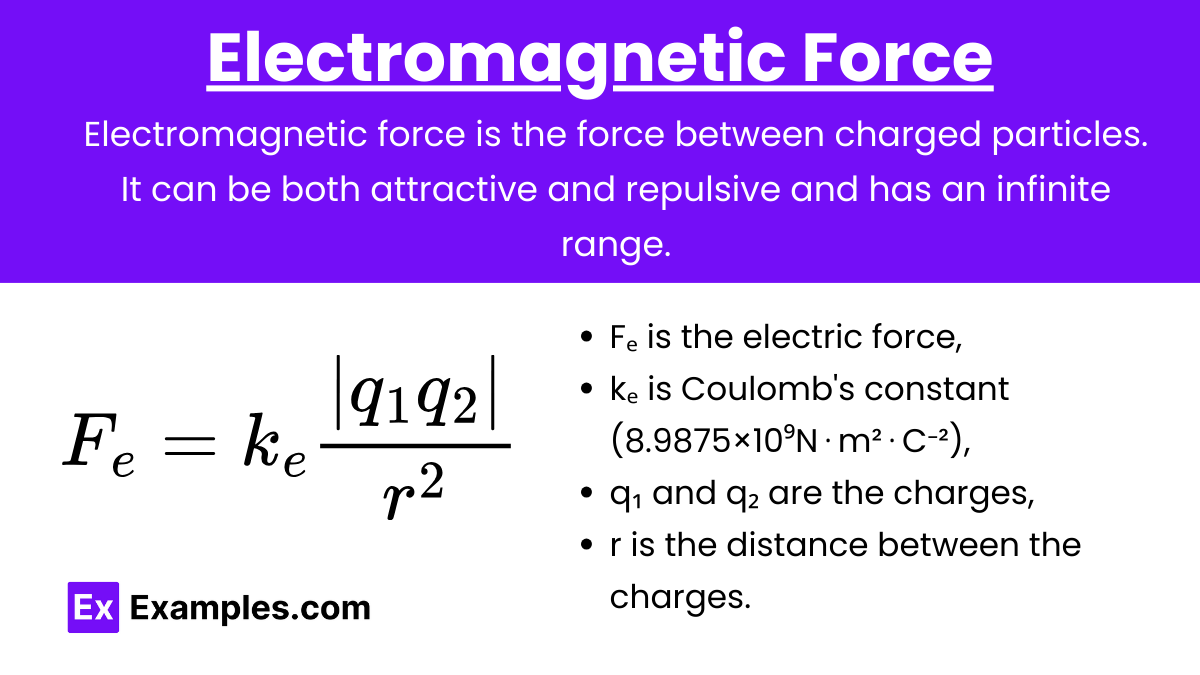
Definition: Electromagnetic force is the force between charged particles. It can be both attractive and repulsive and has an infinite range.
Coulomb's Law: F_e = k_e \frac{|q_1 q_2|}{r^2}
where:
Fₑ is the electric force,
kₑ is Coulomb's constant (8.9875×10⁹N⋅m²⋅C⁻²),
q₁ and q₂ are the charges,
r is the distance between the charges.
Key Points:
Responsible for electricity, magnetism, and light.
Much stronger than gravitational force.
Acts over macroscopic and microscopic scales.
3. Weak Nuclear Force
Definition: The weak nuclear force is responsible for radioactive decay and neutrino interactions. It acts over a very short range, typically less than 10⁻¹⁸m.
Key Points:
Governs beta decay in radioactive elements.
Essential for the processes that power the sun and other stars.
Much stronger than gravitational force but weaker than electromagnetic and strong nuclear forces.
4. Strong Nuclear Force
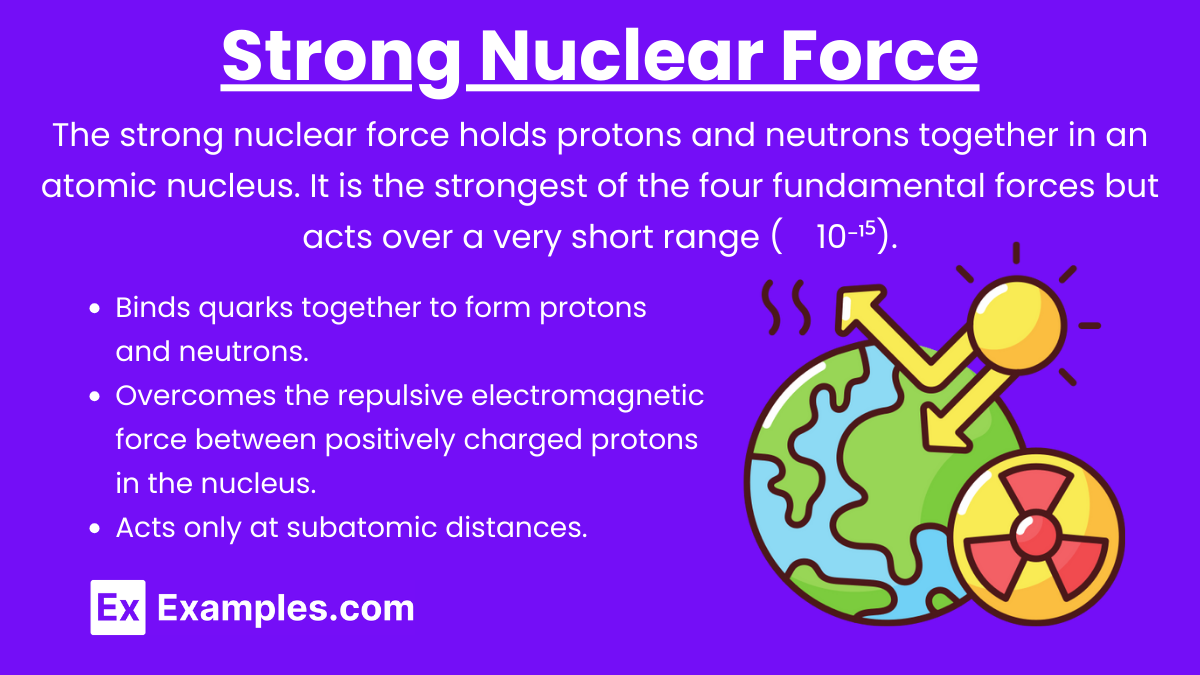
Definition: The strong nuclear force holds protons and neutrons together in an atomic nucleus. It is the strongest of the four fundamental forces but acts over a very short range (∼10⁻¹⁵).
Key Points:
Binds quarks together to form protons and neutrons.
Overcomes the repulsive electromagnetic force between positively charged protons in the nucleus.
Acts only at subatomic distances.
Comparison of Fundamental Forces
Range
Gravitational Force: Infinite
Electromagnetic Force: Infinite
Weak Nuclear Force: Very short (<10⁻¹⁸m)
Strong Nuclear Force: Short (∼10⁻¹⁵m)
Relative Strengths
Gravitational Force: Weakest (10⁻⁴⁰)
Electromagnetic Force: Strong (10⁻²)
Weak Nuclear Force: Weak (10⁻¹³)
Strong Nuclear Force: Strongest (1)
Examples
Gravitational Force
Example: Calculate the gravitational force between Earth (5.972×10²⁴kg) and a 1000 kg satellite 400 km above the Earth's surface.
Solution: \begin{aligned} r &= R_{\text{Earth}} + 400 \, \text{km} = 6.371 \times 10^6 \, \text{m} + 400 \times 10^3 \, \text{m} = 6.771 \times 10^6 \, \text{m} \\ F_g &= \frac{6.674 \times 10^{-11} \times 5.972 \times 10^{24} \times 1000}{(6.771 \times 10^6)^2} \approx 8.79 \times 10^3 \, \text{N} \end{aligned}
Electromagnetic Force
Example: Calculate the force between two electrons (q=−1.6×10⁻¹⁹C) separated by 10⁻¹⁰m.
Solution: F_e = 8.9875 \times 10^9 \frac{(1.6 \times 10^{-19})^2}{(10^{-10})^2} \approx 2.3 \times 10^{-8} \, \text{N}
Weak Nuclear Force
Example: Explain the role of the weak nuclear force in beta decay.
Explanation: In beta decay, a neutron decays into a proton, an electron, and an antineutrino due to the weak nuclear force. The process changes a down quark into an up quark, mediated by the W boson.
Strong Nuclear Force
Example: Explain the role of the strong nuclear force in holding the nucleus together.
Explanation: The strong nuclear force binds protons and neutrons in the nucleus, overcoming the repulsive electromagnetic force between protons. It is responsible for the stability of atomic nuclei.