Understanding open and closed systems is crucial for mastering energy concepts in the AP Physics exam. These systems define how energy and matter interact with their surroundings. Below are detailed notes to help you achieve a high score on your AP Physics exam.
Free AP Physics 1: Algebra-Based Practice Test
Learning Objectives
In the topic of Open and Closed Systems: Energy for the AP Physics exam, you should learn to distinguish between open and closed systems, understand how energy transfers and transformations occur in each, and apply the laws of thermodynamics to analyze these systems. You will explore the concepts of energy conservation, work, and heat transfer, and learn to solve problems involving these principles. Mastery includes recognizing real-world examples and calculating energy changes within both open and closed systems.
Systems
Open Systems
Open System: An open system is one that can exchange both energy and matter with its surroundings. Examples include a boiling pot of water, where steam (matter) and heat (energy) are exchanged with the environment.
Closed Systems
Closed System: A closed system is one that can exchange energy but not matter with its surroundings. An example is a sealed thermos bottle, which can exchange heat with the environment but not matter.
Energy in Open Systems
In an open system, energy transfer can occur in several forms:
Heat Transfer: The system can gain or lose heat to its surroundings.
Work: The system can do work on its surroundings or have work done on it.
Mass Transfer: Matter entering or leaving the system carries energy with it.
Examples of Open Systems:
Human Body: Exchanges heat, does work, and gains/loses mass through food, water, and waste.
Car Engine: Exchanges heat with the environment, does work to move the car, and consumes fuel while emitting exhaust gases.
Ecosystem: Plants absorb sunlight (energy) and carbon dioxide (matter), and animals consume food and excrete waste.
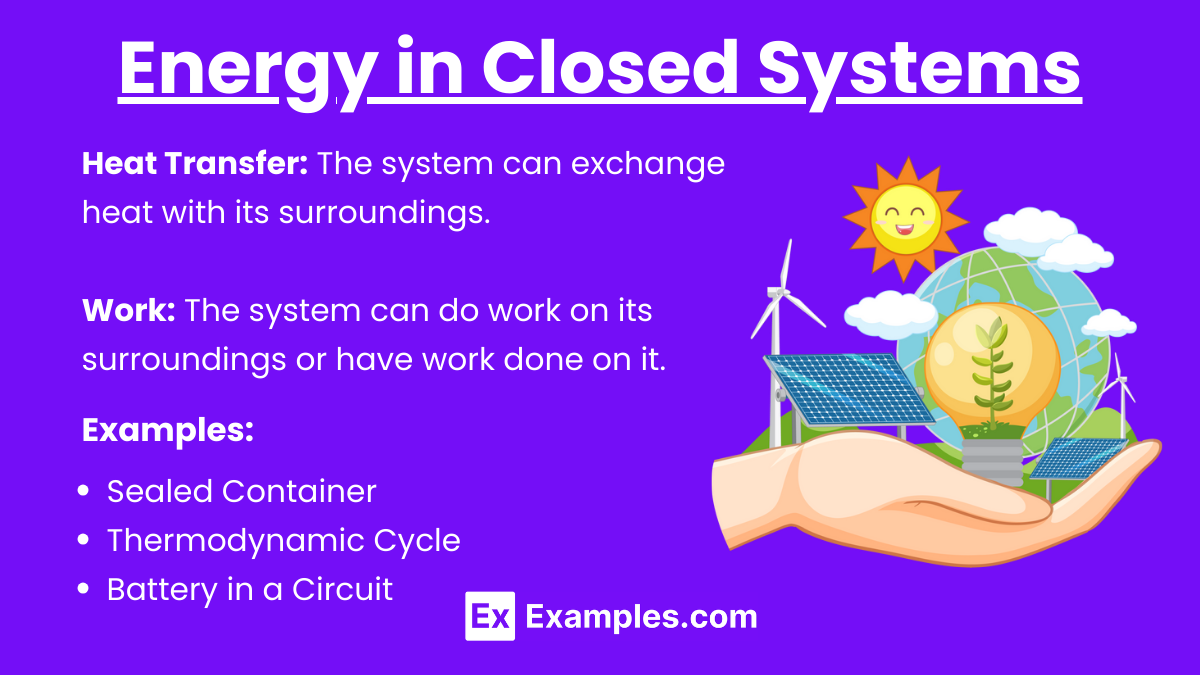
Energy in Closed Systems
In a closed system, energy transfer is limited to:
Heat Transfer: The system can exchange heat with its surroundings.
Work: The system can do work on its surroundings or have work done on it.
Examples of Closed Systems:
Sealed Container: Can exchange heat with the environment but no matter enters or leaves.
Thermodynamic Cycle (e.g., Carnot engine): Exchanges heat with reservoirs and does work without exchanging matter.
Battery in a Circuit: Transfers electrical energy but does not exchange matter.
Energy in Systems
Energy Exchange
Open Systems:
Exchange both energy and matter with their surroundings.
Energy transfer can occur through heat, work, and mass transfer.
Closed Systems:
Exchange only energy with their surroundings, not matter.
Energy transfer occurs through heat and work.
Conservation of Energy
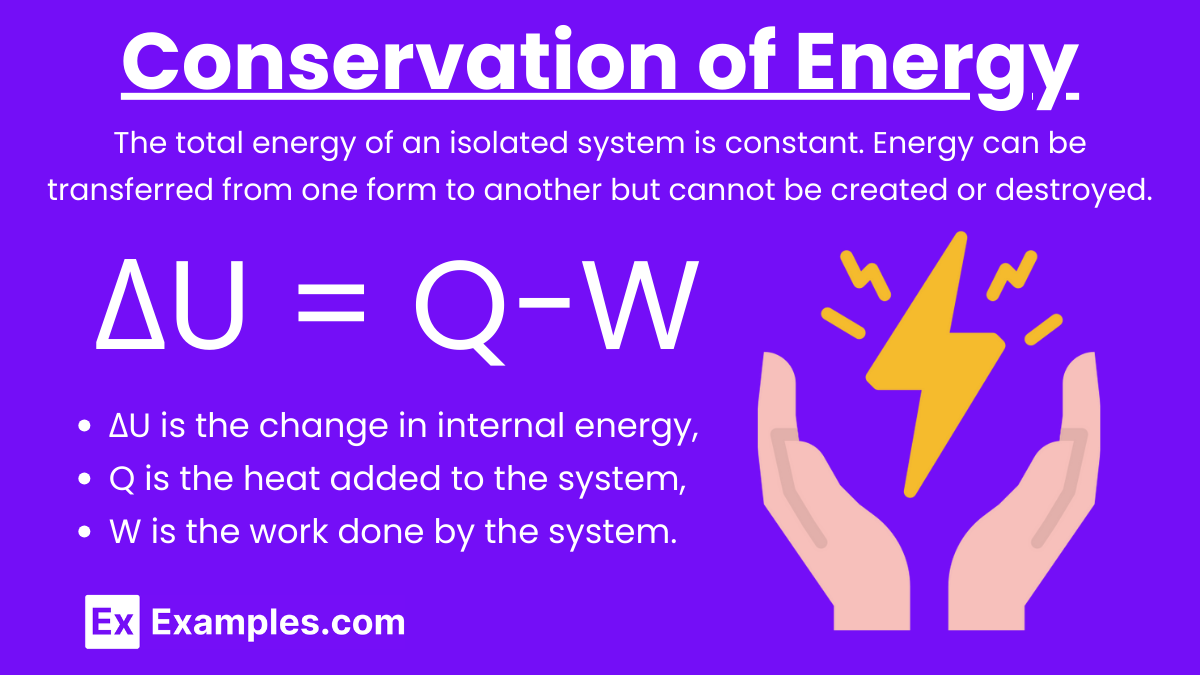
First Law of Thermodynamics: The total energy of an isolated system is constant. Energy can be transferred from one form to another but cannot be created or destroyed.
Equation for Closed Systems: ΔU = Q−W
where:
ΔU is the change in internal energy,
Q is the heat added to the system,
W is the work done by the system.
Internal Energy
Internal Energy (U): The total energy contained within a system, including kinetic and potential energy at the molecular level.
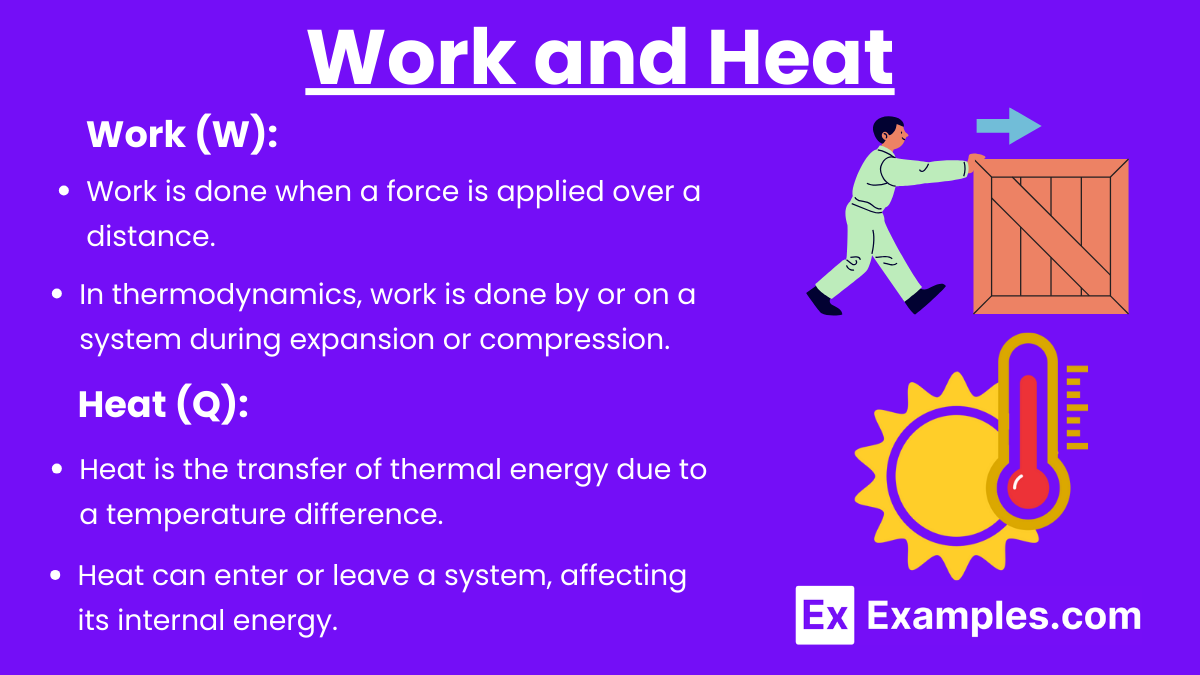
Work and Heat
Work (W):
Work is done when a force is applied over a distance.
In thermodynamics, work is done by or on a system during expansion or compression.
Heat (Q):
Heat is the transfer of thermal energy due to a temperature difference.
Heat can enter or leave a system, affecting its internal energy.
Example 1
Boiling Water in an Open Pot
Scenario: A pot of water is heated on a stove. Steam escapes from the pot.
Analysis:
The system (pot of water) exchanges both heat (energy) and steam (matter) with the surroundings.
It is an open system.
Example 2
Heating Water in a Sealed Container
Scenario: A sealed container of water is heated. No steam escapes.
Analysis:
The system exchanges heat (energy) but not matter with the surroundings.
It is a closed system.
Energy changes the internal energy of the water.
Applications of Open and Closed Systems
Engineering
Open Systems: HVAC systems, where air (matter) and energy are exchanged to control temperature and humidity.
Closed Systems: Refrigerators, where the refrigerant cycles within a closed loop, exchanging heat but not matter with the surroundings.
Environmental Science
Open Systems: Earth's ecosystems, which receive solar energy and exchange matter (e.g., oxygen, carbon dioxide) with the atmosphere.
Closed Systems: Planetary orbits, where planets exchange energy (via gravitational interactions) but no matter is exchanged between them.
Thermodynamics
Open Systems: Steam turbines in power plants, where steam enters and exits, transferring energy to produce electricity.
Closed Systems: Piston-cylinder assemblies in engines, where the gas inside exchanges heat and does work without exchanging matter.