Understanding the definition and conservation of electric charge is crucial for mastering the principles of electricity and magnetism in the AP Physics exam. These concepts are fundamental to analyzing electric interactions and understanding how charges behave in various scenarios. Below are detailed notes along with five examples to help you achieve a high score on your AP Physics exam.
Free AP Physics 2: Algebra-Based Practice Test
Learning Objectives
On the topic of the definition and conservation of electric charge for the AP Physics exam, you should learn that electric charge is a fundamental property of matter that can be either positive or negative. Understand that charge is quantized and conserved, meaning the total charge in an isolated system remains constant. You should also be able to explain how charge can be transferred through processes like conduction and induction, and recognize the role of electrons and protons in these processes.
Electric Charge
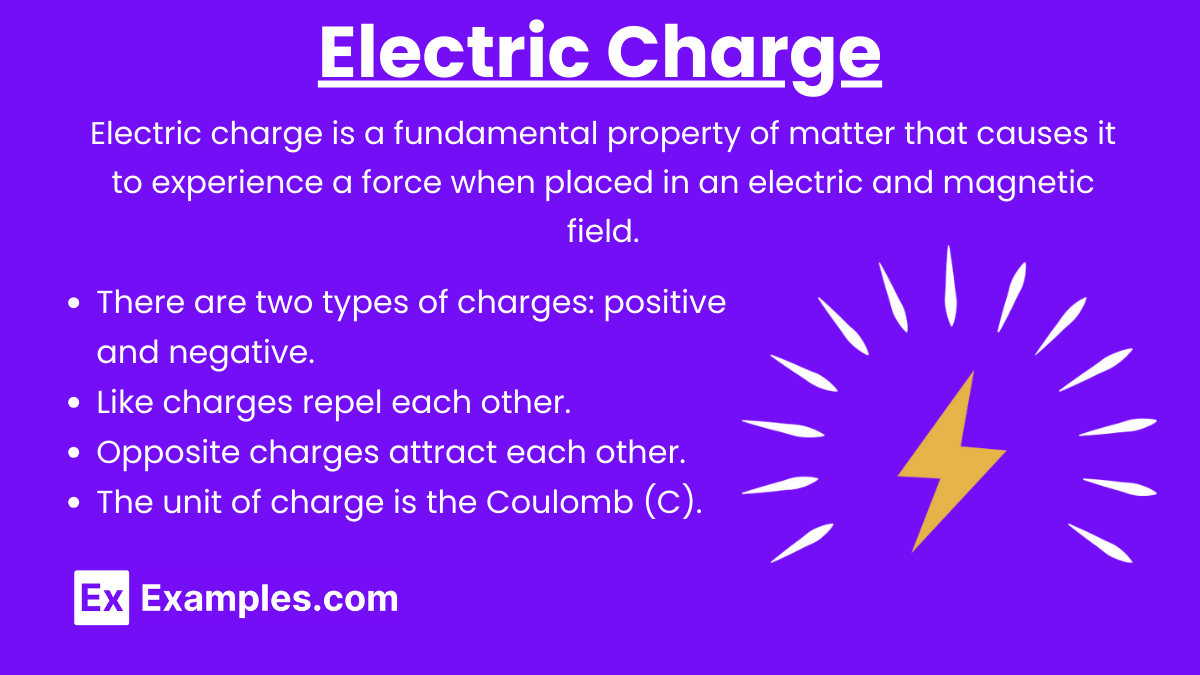
Electric Charge (q): Electric charge is a fundamental property of matter that causes it to experience a force when placed in an electric and magnetic field. There are two types of charges: positive and negative.
Key Points:
Like charges repel each other.
Opposite charges attract each other.
The unit of charge is the Coulomb (C).
The elementary charge eee is the smallest unit of charge, where: e=1.602×10⁻¹⁹C
Quantization of Charge
Quantization of Charge: Charge is quantized, meaning it occurs in discrete amounts. The charge of any object is an integer multiple of the elementary charge.
Conservation of Charge
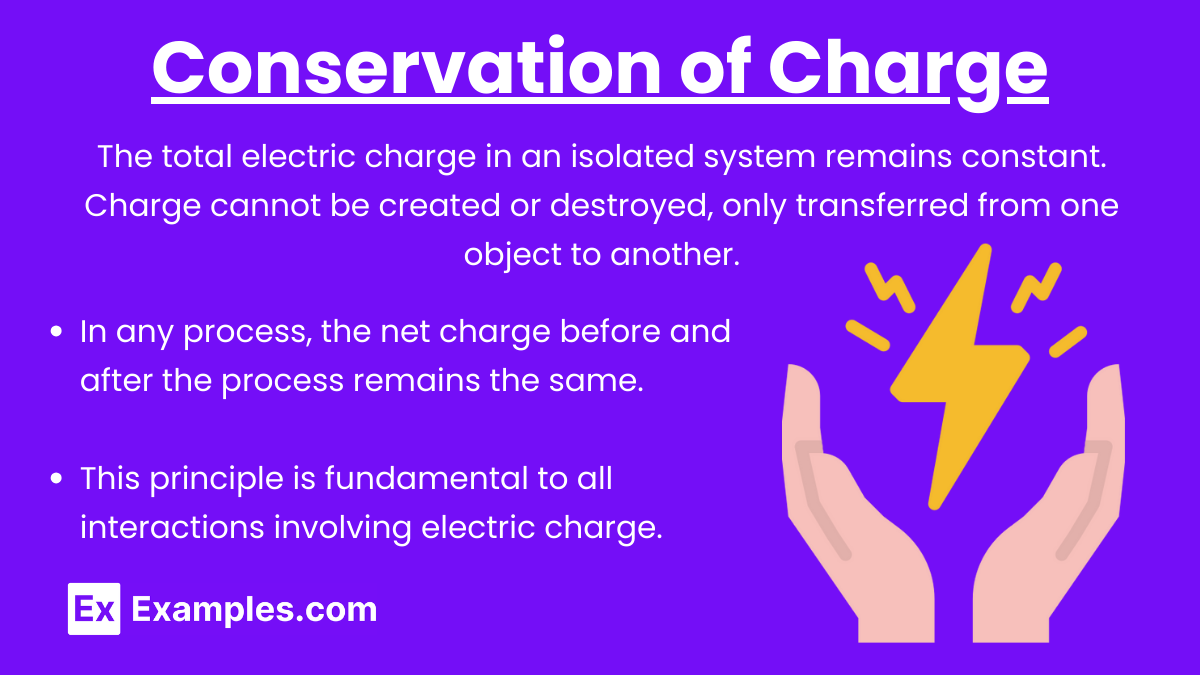
Conservation of Charge: The total electric charge in an isolated system remains constant. Charge cannot be created or destroyed, only transferred from one object to another.
Key Points:
In any process, the net charge before and after the process remains the same.
This principle is fundamental to all interactions involving electric charge.
Properties of Electric Charge
Quantization of Charge: Charge comes in discrete amounts, with the smallest unit being the charge of an electron or proton, approximately 1.6×10⁻¹⁹ coulombs.
Additivity of Charge: The total charge of a system is the algebraic sum of all individual charges within the system.
Invariance of Charge: The charge of a particle does not change with its state of motion.
Charge Conservation: Charge cannot be created or destroyed, only transferred from one body to another.
Example 1
Charging by Friction
Scenario: A glass rod is rubbed with silk, and it becomes positively charged. Explain the process in terms of charge transfer and conservation of charge.
Explanation: When the glass rod is rubbed with silk, electrons are transferred from the glass rod to the silk. The glass rod loses electrons and becomes positively charged, while the silk gains electrons and becomes negatively charged. The total charge before and after rubbing remains zero, demonstrating the conservation of charge.
Example 2
Charging by Conduction
Scenario: A negatively charged rod is brought into contact with a neutral metal sphere. Describe the transfer of charge and the final charge distribution.
Explanation: When the negatively charged rod touches the neutral metal sphere, electrons from the rod move to the sphere. Both the rod and the sphere end up with some negative charge. The total charge in the system is conserved. If the rod initially had −6e of charge and the sphere was neutral, after contact, the rod might have −3e and the sphere −3e, ensuring the total charge is still −6e.
Example 3
Charging by Induction
Scenario: A positively charged rod is brought near a neutral metal sphere without touching it. The sphere is then grounded. Explain the process and the final charge on the sphere.
Explanation: When the positively charged rod is brought near the neutral sphere, electrons in the sphere are attracted toward the rod, causing a negative charge to accumulate near the rod and a positive charge to accumulate on the far side. When the sphere is grounded, electrons from the ground move into the sphere to neutralize the positive charge. After removing the ground and the rod, the sphere is left with a net negative charge. The total charge in the system remains conserved.
Example 4
Conservation of Charge in a Chemical Reaction
Scenario: During an electrolysis reaction, copper ions (Cu²⁺) in a solution gain electrons to form neutral copper atoms. Write the reaction and explain the conservation of charge.
Explanation: The reaction at the cathode can be written as: Cu²⁺ + 2ᵉ⁻ →Cu
Two electrons are gained by each copper ion to form a neutral copper atom. The total charge before the reaction (Cu²⁺ with +2 charge and 2 electrons with -2 charge) is zero, and the total charge after the reaction (neutral copper atom) is also zero, demonstrating the conservation of charge.
Example 5
Conservation of Charge in Radioactive Decay
Scenario: In the beta decay of a neutron, a neutron (n) decays into a proton (p), an electron (e−), and an antineutrino (νˉₑ). Write the decay equation and explain the conservation of charge.
Explanation: The beta decay equation is: n→p+e⁻+νˉₑ
Before the decay, the neutron is neutral (charge 0). After the decay, the proton has a charge of +1, the electron has a charge of -1, and the antineutrino is neutral (charge 0). The total charge before and after the decay is conserved, remaining zero.
Practice Problems
Question 1
Which of the following best describes the principle of conservation of electric charge?
A) Electric charge can be created and destroyed.
B) The total electric charge in an isolated system remains constant.
C) Positive charges can be converted into negative charges.
D) The total electric charge in an isolated system can change if external forces act on it.
Answer: B) The total electric charge in an isolated system remains constant.
Explanation:
The principle of conservation of electric charge states that the total electric charge in an isolated system remains constant regardless of any internal changes that may occur. This means that charge can neither be created nor destroyed, only transferred from one part of the system to another.
Question 2
If a neutral object suddenly gains 5 Coulombs of negative charge, what happens to the system to ensure charge conservation?
A) The object must have initially had a positive charge.
B) Another part of the system must lose 5 Coulombs of positive charge.
C) Another part of the system must gain 5 Coulombs of positive charge.
D) The object will spontaneously lose the gained charge.
Answer: C) Another part of the system must gain 5 Coulombs of positive charge.
Explanation:
According to the conservation of electric charge, if an object gains 5 Coulombs of negative charge, another part of the system must gain an equal amount of positive charge to ensure that the total charge remains constant. This balance maintains the net charge of the system.
Question 3
What is the definition of an electric charge?
A) A measure of the energy stored in an electric field.
B) A fundamental property of particles that causes them to experience a force when placed in an electric and magnetic field.
C) The amount of electric current flowing through a conductor.
D) A measure of the potential difference between two points in an electric field.
Answer: B) A fundamental property of particles that causes them to experience a force when placed in an electric and magnetic field.
Explanation:
Electric charge is a fundamental property of particles, such as electrons and protons, which causes them to experience a force when placed in an electric and magnetic field. Charges can be positive or negative, and like charges repel while opposite charges attract.