The photoelectric effect occurs when light strikes a material and ejects electrons from its surface. This phenomenon, explained by Albert Einstein, demonstrates the quantum nature of light. In AP Physics, understanding the photoelectric effect involves grasping concepts like photons, work function, and threshold frequency. You'll learn how the energy of photons relates to the emission of electrons and the significance of this effect in confirming the particle theory of light, essential for mastering quantum mechanics.
Free AP Physics 2: Algebra-Based Practice Test
Learning Objectives
Understand the principles of the photoelectric effect, including the concepts of photons, work function, and threshold frequency. Learn to apply the photoelectric equation and relate it to experimental observations, such as the effect of light intensity and frequency on electron emission. Be able to explain Einstein's contributions and the significance of the photoelectric effect in demonstrating the particle nature of light. Also, understand practical applications and the role of stopping potential.
Photoelectric Effect
The photoelectric effect is a phenomenon in which electrons are emitted from the surface of a material when it absorbs light. This effect provided crucial evidence for the quantum nature of light and played a pivotal role in the development of quantum mechanics.
History and Discovery
The photoelectric effect was first observed by Heinrich Hertz in 1887. However, it was Albert Einstein who provided a theoretical explanation in 1905, for which he was awarded the Nobel Prize in Physics in 1921. Einstein proposed that light consists of particles called photons and that each photon carries a discrete amount of energy proportional to its frequency.
Key Concepts
Photons and Energy
Photon: A particle of light which carries energy E.
Energy of a Photon: Given by E=hf, where hhh is Planck's constant (6.626×10⁻³⁴ Js) and f is the frequency of the light.
Work Function
Work Function (ϕ): The minimum energy required to eject an electron from the surface of a material.
The work function is specific to each material and represents the binding energy of electrons in the material.
Threshold Frequency
Threshold Frequency (f0): The minimum frequency of light required to cause the ejection of electrons from a material.
Relationship with Work Function: ϕ=hf0.
Photoelectric Equation
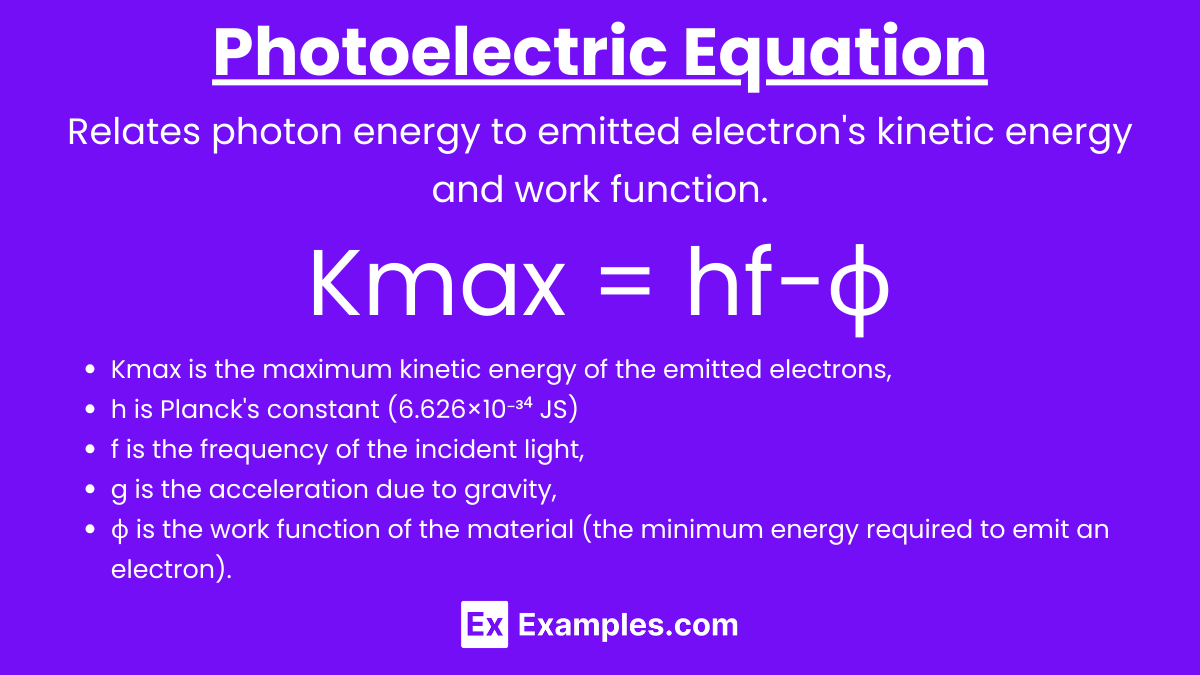
Einstein's photoelectric equation relates the kinetic energy of the emitted electrons to the energy of the incident photons: Kmax=hf−ϕ where Kmax is the maximum kinetic energy of the emitted electrons.
Experimental Observations
Instantaneous Emission: Electrons are emitted immediately after the surface is exposed to light, without any noticeable delay.
Intensity and Kinetic Energy: Increasing the intensity of light increases the number of emitted electrons but does not affect their kinetic energy. The kinetic energy depends only on the frequency of the incident light.
Frequency Dependence: Electrons are emitted only if the frequency of the incident light is above a certain threshold frequency, regardless of the intensity of light.
Experimental Setup

A typical photoelectric effect experiment includes:
Light Source: Emits monochromatic light of variable frequency.
Photocathode: A material that emits electrons when illuminated.
Anode: Collects the emitted electrons.
Vacuum Tube: Ensures electrons travel without interference.
Ammeter: Measures the current produced by the flow of electrons.
Variable Voltage Source: Adjusts the stopping potential to measure the kinetic energy of the emitted electrons.
Stopping Potential
The stopping potential (V0) is the voltage required to stop the most energetic emitted electrons from reaching the anode. It is related to the maximum kinetic energy of the electrons: eV0=Kmax where e is the elementary charge (1.602×10⁻¹⁹ C).
Applications
Photocells: Devices that convert light energy into electrical energy, used in solar panels, light meters, and safety alarms.
Photomultiplier Tubes: Used in medical imaging, nuclear physics, and astrophysics to detect low levels of light.
Automatic Doors and Sensors: Use photoelectric sensors to detect the presence of objects or people.
Important Equations and Constants
Planck's Constant (hhh): 6.626×10⁻³⁴ Js
Elementary Charge (eee): 1.602×10⁻¹⁹ C
Speed of Light (ccc): 3×10⁸m/s
Summary of Equations
Photon Energy: E=hf
Work Function: ϕ=hf0
Photoelectric Equation: Kmax=hf−ϕ
Stopping Potential: eV0=Kmax
Examples of Photoelectric Effect
Solar Panels: Convert sunlight into electricity by using the photoelectric effect to generate a flow of electrons.
Automatic Doors: Use photoelectric sensors to detect the presence of a person or object, triggering the door to open.
Photomultiplier Tubes: Amplify low levels of light in applications like medical imaging and astrophysics.
Light Meters: Measure the intensity of light in photography, relying on the photoelectric effect to provide accurate readings.
Safety Sensors: Used in industrial machines to detect the presence of operators and prevent accidents by stopping the machine when someone is too close.
Practice Test Questions on Photoelectric Effect
Which of the following is necessary for the photoelectric effect to occur?
a) High intensity of light
b) High frequency of light
c) Long wavelength of light
d) Low energy photons
Answer: b) High frequency of light
Explanation: The photoelectric effect requires light with a frequency above the threshold frequency specific to the material. High-frequency light has sufficient energy to overcome the work function of the material and eject electrons, regardless of the light’s intensity.
What happens to the kinetic energy of the emitted electrons if the frequency of the incident light increases?
a) It decreases
b) It remains constant
c) It increases
d) It becomes zero
Answer: c) It increases
Explanation: According to the photoelectric equation Kmax=hf−ϕ, as the frequency fff of the incident light increases, the energy of the photons increases. This results in higher kinetic energy for the emitted electrons, provided the frequency is above the threshold frequency.
If the intensity of light incident on a metal surface is increased while keeping the frequency constant and above the threshold frequency, what will be the effect on the emitted electrons?
a) Number of emitted electrons increases
b) Kinetic energy of emitted electrons increases
c) Number of emitted electrons decreases
d) Threshold frequency increases
Answer: a) Number of emitted electrons increases
Explanation: Increasing the intensity of light increases the number of photons striking the surface per unit time, which leads to an increase in the number of emitted electrons. However, the kinetic energy of the emitted electrons remains unchanged since it depends on the frequency of the light, not the intensity.