Understanding systems and fundamental forces is crucial in physics. A system is a defined portion of the universe for study, and it can be isolated, closed, or open. In AP Physics, mastering the four fundamental forces—gravitational, electromagnetic, weak nuclear, and strong nuclear—is essential. These forces govern interactions at all scales, from cosmic movements to subatomic particles. Grasping their properties and applications forms the foundation for analyzing physical phenomena and solving complex problems in physics.
Learning Objectives
Understand the definition and types of systems (isolated, closed, and open). Comprehend the four fundamental forces—gravitational, electromagnetic, weak nuclear, and strong nuclear—including their properties, formulas, and applications. Grasp how these forces influence various physical phenomena, from planetary motion to atomic interactions. Master key formulas and practice calculations involving these forces to solve related problems accurately. Develop a strong conceptual and analytical understanding of these fundamental concepts.
Systems

system refers to a specific portion of matter in a given region of space that has been selected for study. The system is separated from the rest of the universe (the surroundings) by a boundary, which can be either real or imaginary.
Types of Systems
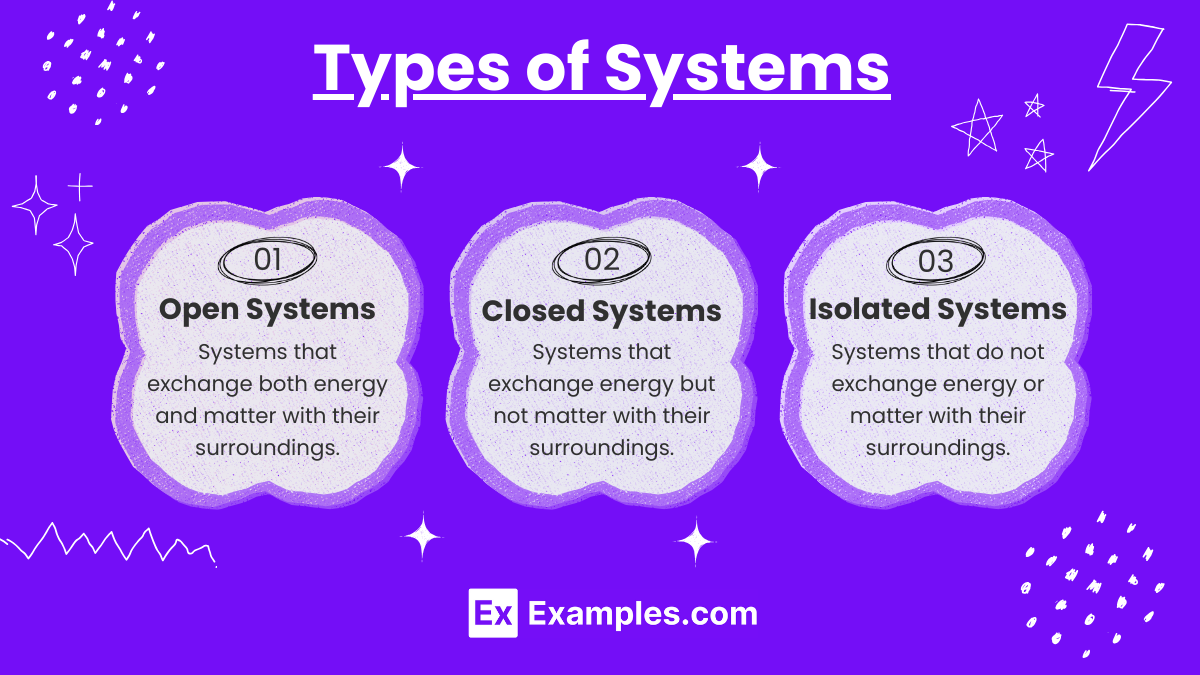
- Isolated Systems
- No exchange of matter or energy with surroundings.
- Example: A thermos flask.
- Closed Systems
- Exchange of energy but not matter with surroundings.
- Example: A sealed, heated container.
- Open Systems
- Exchange of both energy and matter with surroundings.
- Example: A boiling pot of water.
Fundamental Forces
There are four fundamental forces in nature that govern interactions between particles and objects:
1. Gravitational Force
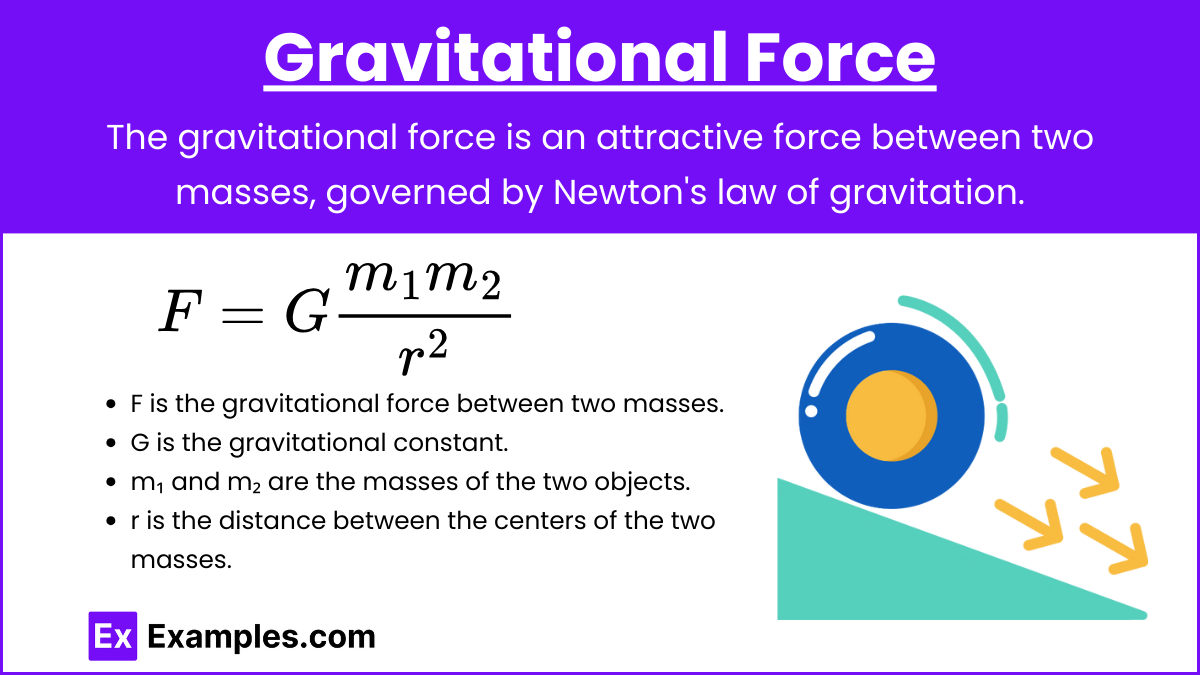
Definition: The attractive force between two masses.
Formula:
![]()
Where:
- F is the gravitational force between two masses.
- G is the gravitational constant.
- m₁ and m₂ are the masses of the two objects.
- r is the distance between the centers of the two masses.
Key Characteristics:
- Always attractive.
- Weakest of the four forces.
- Infinite range.
Examples:
- The force that keeps planets in orbit around the sun.
- The force that causes an apple to fall to the ground.
2. Electromagnetic Force
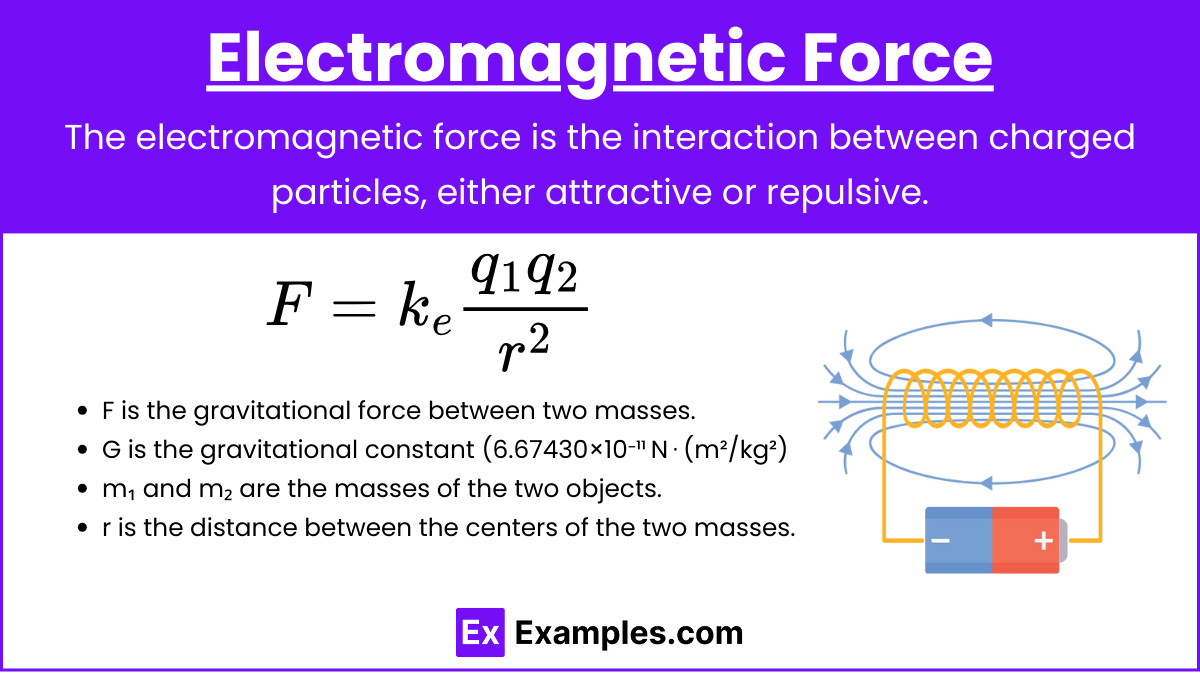
Definition: The force between charged particles.
Formula:
![]()
Where:
- F is the gravitational force between two masses.
- G is the gravitational constant (6.67430×10⁻¹¹ N⋅(m²/kg²)
- m₁ and m₂ are the masses of the two objects.
- r is the distance between the centers of the two masses.
- ke is Coulomb’s constant.
Key Characteristics:
- Can be attractive or repulsive.
- Much stronger than gravity.
- Infinite range.
Examples:
- The force between electrons and protons.
- The force that causes a magnet to attract iron.
3. Weak Nuclear Force

- Definition: A force responsible for radioactive decay and neutrino interactions.
- Key Characteristics:
- Short range (about 0.1% of the diameter of a typical atomic nucleus).
- Responsible for beta decay in radioactive elements.
- Much stronger than gravity but weaker than the electromagnetic and strong nuclear forces.
- Examples:
- Beta decay in radioactive elements.
- Processes in the sun that lead to fusion.
4. Strong Nuclear Force
- Definition: The force that holds protons and neutrons together in an atomic nucleus.
- Key Characteristics:
- Strongest of the four forces.
- Very short range (about the size of an atomic nucleus).
- Attractive force.
- Examples:
- Binding protons and neutrons in the nucleus.
- Responsible for the energy released in nuclear fission and fusion.
Examples of Systems and Fundamental Forces
- Planetary System and Gravitational Force
- The gravitational force keeps planets in orbit around the sun.
- Electromagnetic Force in Circuits
- The force between electrons and protons enables electric current flow in circuits.
- Nuclear Reactor and Strong Nuclear Force
- The strong nuclear force binds protons and neutrons, allowing nuclear fission reactions.
- Radioactive Decay and Weak Nuclear Force
- The weak nuclear force is responsible for beta decay in radioactive elements.
- Boiling Water in an Open System
- An open system where water exchanges heat and vapor with the surroundings.
Practice Test Questions on Systems and Fundamental Forces
Question 1: Which of the following best describes an isolated system?
- A) A system that exchanges both energy and matter with its surroundings.
- B) A system that exchanges only energy with its surroundings.
- C) A system that exchanges neither energy nor matter with its surroundings.
- D) A system that exchanges only matter with its surroundings.
Answer: C) A system that exchanges neither energy nor matter with its surroundings.
Explanation: An isolated system is one where neither energy nor matter is exchanged with the surroundings. This is the key characteristic that differentiates it from closed (energy exchange only) and open (both energy and matter exchange) systems.
Question 2: What is the primary role of the strong nuclear force?
- A) To keep electrons in orbit around the nucleus.
- B) To bind protons and neutrons within the atomic nucleus.
- C) To cause radioactive decay in unstable nuclei.
- D) To attract objects with mass toward each other.
Answer: B) To bind protons and neutrons within the atomic nucleus.
Explanation: The strong nuclear force is the fundamental force responsible for holding protons and neutrons together in the nucleus, despite the repulsive electromagnetic force between the positively charged protons. It is the strongest of the four fundamental forces but operates over very short distances.
Question 3: Which of the following interactions is governed by the weak nuclear force?
- A) Gravitational attraction between two planets.
- B) Electromagnetic repulsion between two electrons.
- C) The decay of a neutron into a proton, electron, and antineutrino.
- D) The fusion of hydrogen nuclei in the sun.
Answer: C) The decay of a neutron into a proton, electron, and antineutrino.
Explanation: The weak nuclear force is responsible for beta decay, a type of radioactive decay where a neutron decays into a proton, an electron, and an antineutrino. This force operates at a subatomic level and is weaker than both the strong nuclear force and the electromagnetic force.