Understanding thermodynamic systems is crucial for mastering concepts in thermodynamics, which are essential for the AP Physics exam. Thermodynamics deals with heat, work, temperature, and the statistical behaviors of systems. Below are detailed notes along with five examples to help you achieve a high score on your AP Physics exam.
Free AP Physics 2: Algebra-Based Practice Test
Learning Objectives
In studying thermodynamic systems for the AP Physics exam, you should understand the different types of systems (open, closed, isolated) and their interactions with surroundings. Master the laws of thermodynamics, specifically the conservation of energy, entropy, and heat transfer processes. Gain the ability to analyze work done by and on the system, apply PV diagrams, and solve problems involving thermal efficiency, engines, and refrigerators. Familiarize yourself with real-world applications to solidify theoretical concepts and their practical significance.
Thermodynamic Systems
Thermodynamic System: A thermodynamic system is a specific portion of the physical universe chosen for analysis. Everything outside this system is considered the surroundings. Systems can exchange energy and matter with their surroundings depending on their boundaries.
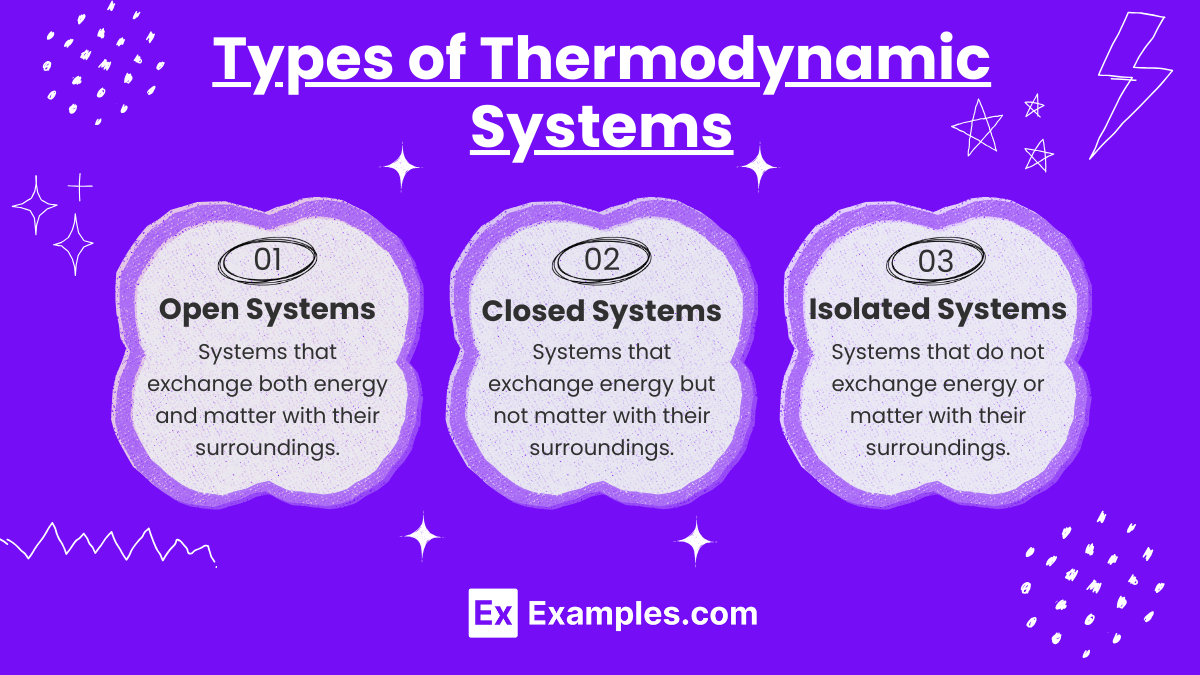
Types of Thermodynamic Systems
Open System:
An open system can exchange both energy and matter with its surroundings.
Example: A boiling pot of water where steam (matter) and heat (energy) are exchanged with the surroundings.
Closed System:
A closed system can exchange energy but not matter with its surroundings.
Example: A sealed, insulated container with gas where heat can be transferred, but the gas cannot escape.
Isolated System:
An isolated system cannot exchange either energy or matter with its surroundings.
Example: A thermos bottle (ideal case) where neither heat nor matter is transferred.
State Variables
State Variables: These are properties that describe the state of a system, such as pressure (P), volume (V), temperature (T), and internal energy (U). They are independent of the path taken to reach that state.
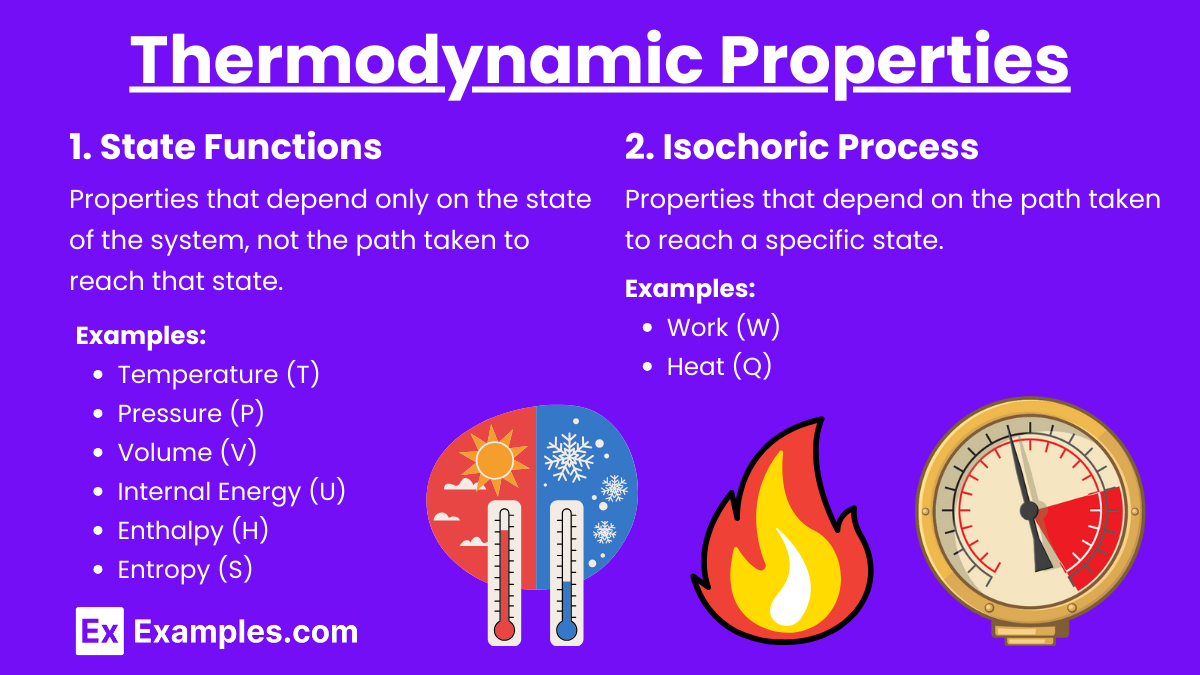
Thermodynamic Properties
1. State Functions
Properties that depend only on the state of the system, not the path taken to reach that state.
Examples:
Temperature (T)
Pressure (P)
Volume (V)
Internal Energy (U)
Enthalpy (H)
Entropy (S)
2. Process Functions
Properties that depend on the path taken to reach a specific state.
Examples:
Work (W)
Heat (Q)
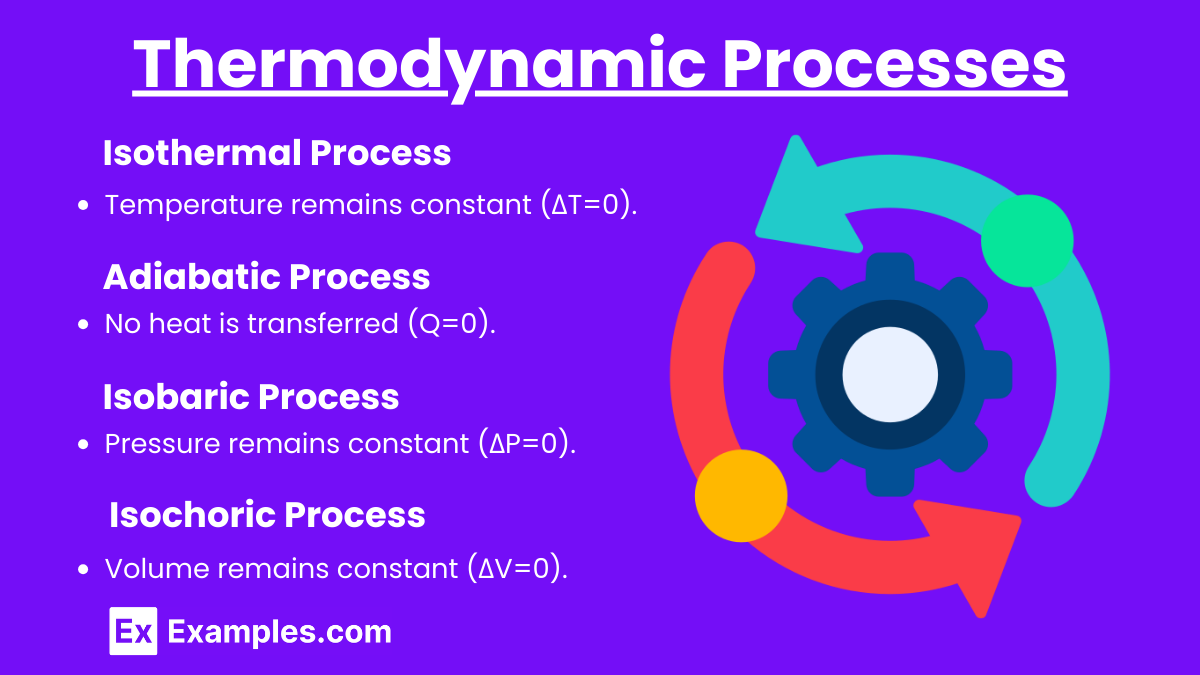
Thermodynamic Processes
1. Isothermal Process
Temperature remains constant (ΔT=0).
Characteristics:
Heat Transfer: Heat is transferred to maintain constant temperature.
Example: Slow compression or expansion of a gas in a piston.
2. Adiabatic Process
No heat is transferred (Q=0).
Characteristics:
Temperature Change: Temperature can change because no heat is exchanged.
Example: Rapid compression or expansion of gas where heat doesn't have time to enter or leave the system.
3. Isobaric Process
Pressure remains constant (ΔP=0).
Characteristics:
Work Done: Work done by the system is proportional to the volume change.
Example: Heating of gas in a piston that can move freely to maintain constant pressure.
4. Isochoric Process
Volume remains constant (ΔV=0).
Characteristics:
Work Done: No work is done as volume doesn’t change.
Example: Heating of gas in a rigid, non-expandable container.
The Laws of Thermodynamics
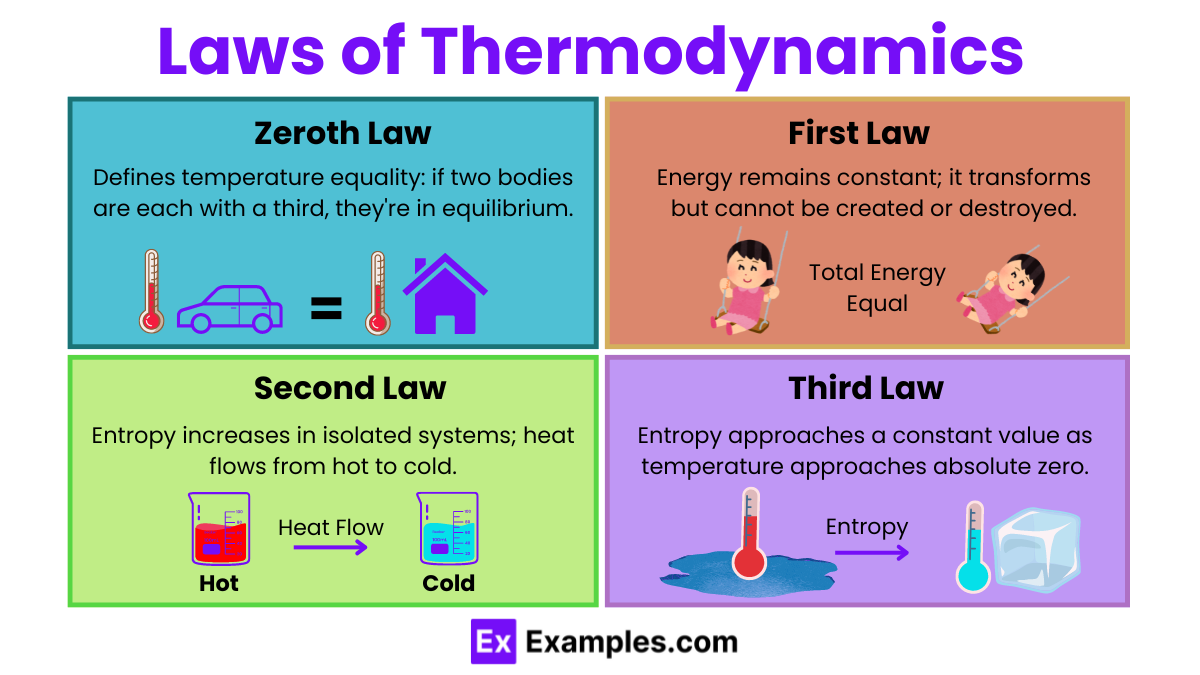
Zeroth Law of Thermodynamics
Zeroth Law: If two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law defines temperature.
First Law of Thermodynamics
First Law: Energy cannot be created or destroyed, only transferred or converted from one form to another. This is also known as the principle of conservation of energy.
Formula: ΔU = Q−W
where:
ΔU is the change in internal energy,
Q is the heat added to the system,
W is the work done by the system.
Second Law of Thermodynamics
Second Law: The total entropy of an isolated system can never decrease over time. In any energy exchange, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state (entropy will increase).
Third Law of Thermodynamics
Third Law: As the temperature of a system approaches absolute zero, the entropy of a perfect crystal approaches a constant minimum.
Examples of Thermodynamic Systems
Example 1: Ideal Gas in a Piston
Scenario: An ideal gas is confined in a piston. When heat is added to the gas, it expands, doing work on the piston.
Analysis:
Type of System: Closed system (exchanges energy but not matter).
Process: Isobaric (constant pressure) expansion.
First Law Application: ΔU = Q−W.
Example 2: Insulated Container with Ice and Water
Scenario: An insulated container holds a mixture of ice and water. The system reaches thermal equilibrium without exchanging heat with its surroundings.
Analysis:
Type of System: Isolated system (no exchange of energy or matter).
Process: Thermal equilibrium.
Outcome: Both ice and water reach the same temperature, and the total energy remains constant.
Example 3: Steam Engine
Scenario: A steam engine operates by converting heat from boiling water into mechanical work.
Analysis:
Type of System: Open system (exchanges energy and matter).
Process: Thermodynamic cycle (heat engine).
First Law Application: Work is done by the system, heat is transferred from the boiler to the engine.
Example 4: Refrigerator
Scenario: A refrigerator removes heat from its interior to maintain a low temperature, expelling the heat to the surroundings.
Analysis:
Type of System: Closed system (exchanges energy but not matter).
Process: Cyclic process (refrigeration cycle).
Second Law Application: Heat is transferred from a cold reservoir to a hot reservoir.
Example 5: Adiabatic Compression in a Gas Cylinder
Scenario: A gas in a cylinder is rapidly compressed, and no heat is exchanged with the surroundings.
Analysis:
Type of System: Closed system (exchanges energy but not matter).
Process: Adiabatic compression (no heat transfer).
First Law Application: ΔU = −W, since Q = 0.
Practice Problems
Question 1:
In an isothermal process, which of the following statements is true about the internal energy of an ideal gas?
A) It increases.
B) It decreases.
C) It remains constant.
D) It fluctuates.
Answer: C) It remains constant.
Explanation:
In an isothermal process, the temperature of the system remains constant. For an ideal gas, the internal energy U is solely a function of temperature. Since the temperature does not change in an isothermal process, the internal energy of the gas also remains constant. Therefore, the correct answer is that the internal energy remains constant.
Question 2:
Which of the following is an example of an adiabatic process?
A) Compressing gas in an insulated piston.
B) Heating water in an open container.
C) Melting ice in a warm room.
D) Boiling water at a constant pressure.
Answer: A) Compressing gas in an insulated piston.
Explanation:
An adiabatic process is one in which no heat is exchanged with the surroundings. This means the system is perfectly insulated. Compressing gas in an insulated piston ensures that there is no heat transfer into or out of the gas, making it an adiabatic process. Heating water in an open container, melting ice, and boiling water all involve heat exchange with the surroundings, so they are not adiabatic processes. Therefore, the correct answer is compressing gas in an insulated piston.
Question 3:
A thermodynamic system undergoes a cyclic process that returns it to its initial state. What is the net change in internal energy of the system after one complete cycle?
A) It increases.
B) It decreases.
C) It remains constant.
D) It depends on the type of process.
Answer: C) It remains constant.
Explanation:
In a cyclic process, a system undergoes a series of changes that eventually return it to its initial state. According to the first law of thermodynamics, the change in internal energy ΔU is given by:
ΔU = Q−W
where Q is the heat added to the system and W is the work done by the system. For a complete cycle, the system returns to its initial state, meaning its internal energy must be the same as it was at the beginning of the cycle. Hence, the net change in internal energy after one complete cycle is zero. Therefore, the correct answer is that the internal energy remains constant.