What are competent cells primarily used for?
Protein synthesis
DNA replication
Genetic transformation
ATP production

Dive into the fascinating world of competent cells, the unsung heroes of molecular biology and genetic engineering. These specially prepared cells are crucial for DNA uptake, enabling the transformation process that underlies cloning, gene expression studies, and much more. Our comprehensive guide introduces you to the science behind competent cells, showcasing their pivotal role in advancing research and biotechnological applications. Explore real-world examples and discover how these cells are the key to unlocking the potential of genetic manipulation and the development of groundbreaking therapies.
Competent cells are a fundamental tool in molecular biology, engineered to take up extraneous DNA from their surroundings through a process known as transformation. This capability is not inherently present in most bacteria; therefore, cells must be made competent through specific treatments that alter the cell membrane’s permeability. Competency allows for the introduction of foreign DNA, such as plasmids, into bacterial cells, facilitating genetic modifications that underpin a wide range of research and biotechnological applications.
The principle of competent cells lies at the heart of modern genetic engineering, serving as a crucial step in the process of bacterial transformation. This principle revolves around the ability of certain bacterial cells to uptake and incorporate foreign DNA from their environment into their own genetic material. By understanding and harnessing this principle, scientists can introduce new genes into bacteria, facilitating a wide range of genetic manipulations for research, industrial, and medical applications. This guide delves into the foundational concepts, techniques, and implications of making and utilizing competent cells.
Competency refers to a cell’s ability to alter its cell wall and membrane structure to take up naked DNA molecules. Naturally, bacterial cells possess a negatively charged cell wall that repels the DNA’s phosphate backbone, also negatively charged. The induction of competency involves temporarily neutralizing these charges and creating pores in the cell membrane, allowing DNA to enter the cell.
In the chemical competence method, a critical step involves exposing the DNA-cell mixture to a brief heat shock. This sudden increase in temperature facilitates the DNA’s entry into the cell by creating thermal imbalances across the cell membrane, enhancing the permeability of the membrane to DNA.
Competent cells are foundational to various genetic engineering tasks, including:
While the principle of competent cells has revolutionized biotechnology, it also raises ethical considerations regarding genetic modification and its implications. Regulatory frameworks and ethical guidelines are essential to ensure responsible use of this technology, balancing innovation with respect for natural biodiversity and human health.
In summary, the principle of competent cells embodies a key technique in molecular biology, enabling the manipulation of genetic material for scientific advancement. By mastering this principle, researchers can unlock the full potential of bacterial systems, contributing to significant breakthroughs in medicine, industry, and environmental science.
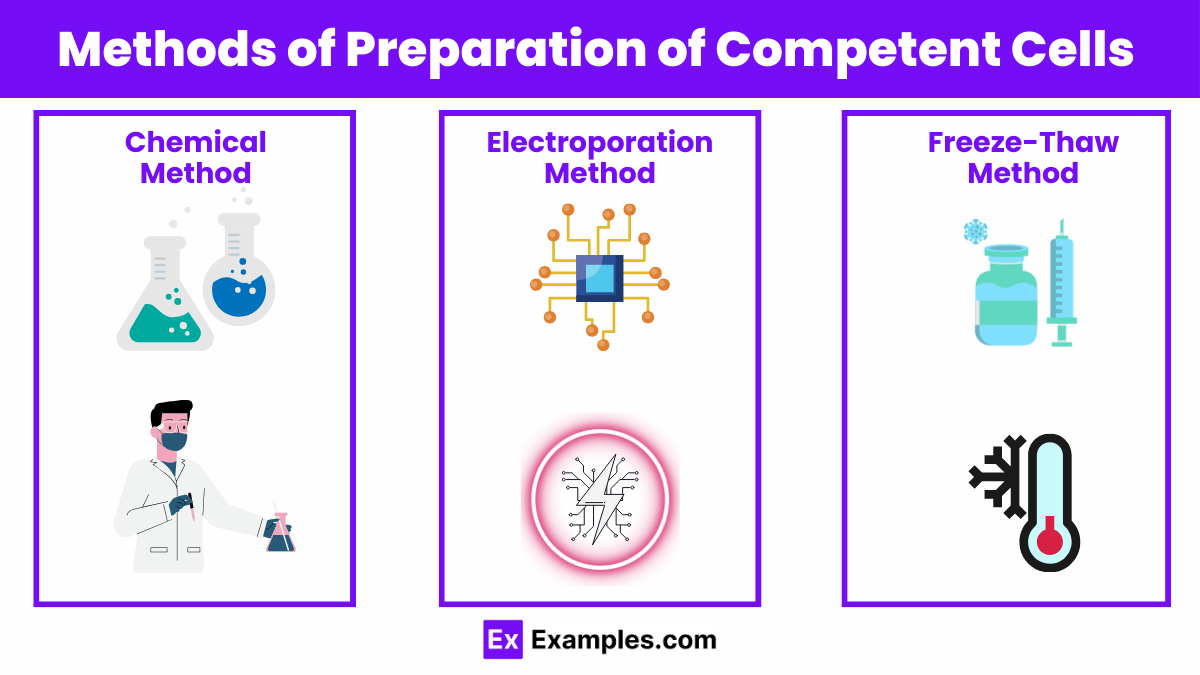
The preparation of competent cells is a pivotal procedure in molecular biology, enabling the transformation of bacteria with foreign DNA for various genetic manipulations. This process involves treating bacterial cells to make their cell walls permeable to DNA uptake. The methods of preparing competent cells vary, each with its own advantages, applications, and efficiencies. This guide explores the most widely used methods, offering insights into the techniques that facilitate the foundational work of genetic engineering.
The chemical method is the most common approach for preparing competent cells, favored for its simplicity and effectiveness. It involves treating bacterial cells, typically Escherichia coli, with a solution of calcium chloride (CaCl2) or other divalent cations like rubidium chloride (RbCl). The treatment increases the permeability of the cell membrane by neutralizing the repulsive forces between the negatively charged DNA and the cell wall.
Electroporation is a technique used for cells that are difficult to transform by chemical methods or for the introduction of large DNA molecules. This method uses an electrical pulse to create temporary pores in the bacterial cell membranes through which DNA can enter.
The freeze-thaw method is a less commonly used technique that exploits the physical disruption of cell membranes through repeated freezing and thawing. This method can be used as a simple, albeit lower efficiency, alternative to chemical and electroporation methods.
In the realm of molecular biology and genetic engineering, competent cells serve as crucial tools for introducing foreign DNA into bacterial cells, facilitating a variety of genetic modifications. The development of different types of competent cells, each tailored for specific applications, has significantly advanced research capabilities. This comprehensive guide explores the main types of competent cells, shedding light on their unique properties, applications, and how they enhance the efficiency of transformation processes.
Chemically competent cells are prepared using a chemical process, typically involving calcium chloride (CaCl2), which increases the cell membrane’s permeability to DNA. This type is widely used for routine cloning procedures due to its simplicity and cost-effectiveness. The process involves incubating bacterial cells with CaCl2 and subjecting them to a heat shock to facilitate DNA uptake. While chemically competent cells are suitable for many standard cloning applications, their transformation efficiency can vary, making them less ideal for applications requiring high efficiency.
Electrocompetent cells are prepared for transformation by electroporation, a method that uses electrical pulses to create temporary pores in the cell membrane, allowing DNA to enter the cell. This preparation method generally yields a higher transformation efficiency compared to chemical methods and is preferred for applications requiring the introduction of large DNA constructs or for use with bacterial strains that are less amenable to chemical transformation. Electrocompetent cells are essential for high-throughput cloning, library construction, and applications requiring high transformation efficiencies.
High-efficiency competent cells are specifically designed to maximize DNA uptake, offering transformation efficiencies that are orders of magnitude higher than standard preparations. These cells are ideal for applications such as the construction of genomic libraries, where the transformation of a large number of different DNA molecules is necessary. High-efficiency competent cells often undergo specialized preparation procedures and optimizations, making them more expensive but invaluable for certain high-precision applications.
Specialized competent cells are engineered to possess specific genetic traits that facilitate particular types of cloning or genetic manipulation.
In the field of genetic engineering and molecular biology, the transformation of bacterial cells with foreign DNA is a fundamental process. This process hinges on the bacterial cells being ‘competent’ — capable of taking up DNA from their environment. Competence can be categorized into two main types: natural and artificial. Understanding the distinction between these two forms is crucial for researchers to select the most appropriate method for their experiments. This guide delves into the differences between natural and artificial competent cells, highlighting their mechanisms, applications, and implications for scientific research.
Natural competence refers to the innate ability of certain bacterial species to take up DNA from their surroundings under specific environmental conditions. This phenomenon is a genetically programmed response that allows bacteria to acquire new genetic traits, contributing to genetic diversity and adaptation. Natural competence is not widespread among bacteria; it is a trait observed in a limited number of species, such as Streptococcus pneumoniae, Neisseria gonorrhoeae, and Bacillus subtilis.
Artificial competence is induced in bacterial cells through laboratory techniques, enabling them to take up DNA regardless of their natural predisposition. This method expands the range of bacteria available for genetic transformation, making it a cornerstone of molecular cloning, genetic engineering, and biotechnology. Artificial competence can be induced in a wide variety of bacterial strains, most commonly Escherichia coli, through chemical treatment or physical methods like electroporation.
Understanding the differences between natural and artificial competent cells is essential for researchers to make informed decisions regarding the best approach for their specific genetic manipulation goals. While natural competence offers valuable insights into bacterial behavior and evolution, artificial competence remains a key technique in the arsenal of molecular biology, driving forward the fields of genetic engineering and biotechnology.
The preparation of chemically competent cells is a pivotal technique in molecular biology, enabling the transformation of bacteria with foreign DNA. This method relies on the use of specific salts and chemicals to permeabilize the bacterial cell wall, facilitating the uptake of plasmid DNA. Understanding the roles of these substances is crucial for optimizing the transformation efficiency and success of genetic engineering projects. This comprehensive guide details the key salts and chemicals involved in making chemically competent cells, outlining their functions and significance in the process.
Calcium chloride is the most commonly used chemical in the preparation of competent cells. CaCl2 treatment alters the cell membrane’s permeability, allowing DNA to enter the cell more easily. The positive charge of calcium ions helps neutralize the repulsion between the negatively charged DNA and bacterial cell wall, facilitating the binding of DNA to the cell surface.
Rubidium chloride is another salt used in the preparation of competent cells, particularly for strains or plasmids that respond poorly to CaCl2 treatment. Similar to calcium chloride, RbCl works by neutralizing the charge repulsion between the cell wall and DNA, but it can offer higher transformation efficiencies for certain applications.
Magnesium chloride is often used in conjunction with CaCl2 in the preparation of competent cells. MgCl2 stabilizes the DNA and cell membrane, enhancing the overall efficiency of DNA uptake. It also plays a crucial role in maintaining the integrity of the cells during the transformation process.
Glycerol is commonly added to the competent cell suspension as a cryoprotectant. It protects cells from damage during freezing and thawing, allowing for the long-term storage of competent cells without significantly reducing their transformation efficiency. Glycerol helps maintain cell membrane fluidity at low temperatures, ensuring cells remain competent after thawing.
Dimethyl sulfoxide is occasionally used in the preparation of competent cells to increase membrane permeability further. DMSO can enhance the uptake of DNA by disrupting the lipid bilayer of the cell membrane. However, its use must be carefully optimized as it can also increase cell mortality.
Manganese chloride is used in some protocols to improve the transformation efficiency of competent cells. MnCl2 acts similarly to MgCl2, stabilizing DNA and enhancing its interaction with the cell membrane. It is particularly useful in protocols aimed at transforming cells with large plasmids or genomic DNA.
Potassium acetate is included in some competent cell preparation protocols to adjust the ionic strength of the solution, which can affect the efficiency of DNA uptake. KOAc helps maintain osmotic balance, preventing cell lysis during the transformation process.
Hexamine cobalt chloride is a less common additive that can be used to increase the efficiency of DNA uptake in certain types of competent cells. It acts by further stabilizing the DNA-cell complex, facilitating the passage of DNA into the cell.
A cell is made competent through methods like chemical treatment with calcium chloride or electroporation, not through natural replication processes or by altering DNA directly.
Being ‘competent’ means bacterial cells are prepared to uptake foreign DNA, a crucial step for successful transformation and genetic manipulation in experiments.
In biotechnology experiments, a cell must be ‘competent’ to efficiently take up foreign DNA for gene expression studies. Calcium ions neutralize the charge repulsion between DNA and the cell wall, facilitating DNA entry.
Competent cells are a fundamental cornerstone in molecular biology, enabling the introduction of foreign DNA into bacterial hosts for genetic manipulation. Through methods like chemical treatment and electroporation, cells are prepared for transformation, paving the way for advancements in genetic engineering, biotechnology, and medical research. Understanding and utilizing competent cells is crucial for the development of novel therapies and the exploration of the genetic basis of life.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What are competent cells primarily used for?
Protein synthesis
DNA replication
Genetic transformation
ATP production
Which of the following methods is commonly used to make bacterial cells competent?
Heat shock
Ultracentrifugation
Gel electrophoresis
PCR
Competent cells are often treated with what chemical to enhance DNA uptake?
Sodium chloride
Calcium chloride
Magnesium sulfate
Potassium phosphate
Which type of DNA is commonly introduced into competent cells?
Mitochondrial DNA
Plasmid DNA
Genomic DNA
Chloroplast DNA
What is the purpose of using ice during the heat shock transformation process?
To activate enzymes
To increase cell division
To stabilize the cell membrane
To lyse the cells
Electroporation is an alternative method to heat shock for making cells competent. What does electroporation involve?
Using high temperature
Using electric pulses
Using chemical reagents
Using UV light
Which of the following is a characteristic of chemically competent cells?
They are resistant to antibiotics
They can naturally take up DNA without treatment
They have been treated with calcium chloride
They have no cell wall
What is the main difference between chemically competent cells and electrocompetent cells?
The type of DNA they can take up
The method used to induce competence
Their ability to grow in culture
Their resistance to heat
After introducing plasmid DNA into competent cells, what is the next step to select transformed cells?
Incubate in nutrient broth
Plate on antibiotic-containing agar
Perform gel electrophoresis
Use a spectrophotometer
Competent cells are often stored at what temperature for long-term use?
4°C
-20°C
-80°C
Room temperature
Before you leave, take our quick quiz to enhance your learning!

