Globular vs Fibrous protein – Differences Explained with Examples
This article explores the distinct characteristics and functions of globular and fibrous proteins, two fundamental protein types crucial to understanding biological structures and processes. Globular proteins, known for their solubility and dynamic roles in metabolic activities, contrast sharply with the robust, structural nature of fibrous proteins. By delving into their molecular configurations, biological roles, and significance in health and disease, we aim to provide a clear differentiation between these proteins, enhancing both academic understanding and practical application in biomedical sciences.
Globular Proteins
Globular proteins are a class of proteins that are generally spherical or globular in shape. They are one of the two main protein classes, with the other being fibrous proteins. The compact, folded structure of globular proteins makes them soluble in water, which is a key distinguishing feature from fibrous proteins.
Characteristics
- Solubility: Globular proteins are typically soluble in aqueous solutions. Their solubility is due to the hydrophilic (water-attracting) amino acid residues that are on the protein’s surface, allowing them to interact with water molecules.
- Structure: They have a highly intricate three-dimensional structure, which includes:
- Primary Structure: The sequence of amino acids in the polypeptide chain.
- Secondary Structure: Local folding into structures such as alpha-helices and beta-pleated sheets.
- Tertiary Structure: The overall three-dimensional shape of the protein, formed by the folding of the secondary structures.
- Quaternary Structure: Some globular proteins consist of multiple polypeptide chains or subunits, held together by various interactions.
- Functionality: Due to their soluble nature and dynamic structures, globular proteins are highly versatile and play various roles in biological processes.
Functions of Globular Proteins
- Enzymes: Many globular proteins function as enzymes, which are biological catalysts that accelerate chemical reactions. For example, amylase helps in the digestion of carbohydrates by breaking down starch into sugars.
- Transport Proteins: These proteins transport substances within an organism. Hemoglobin is a classic example, transporting oxygen from the lungs to tissues and returning carbon dioxide from tissues to the lungs.
- Regulatory Proteins: Globular proteins often act as hormones and other regulatory molecules. For instance, insulin regulates glucose metabolism in the body.
- Defensive Proteins: Antibodies are globular proteins that play a critical role in the immune response by identifying and neutralizing foreign substances like bacteria and viruses.
- Storage Proteins: Some globular proteins store important molecules. Ferritin stores iron in the liver and releases it in a controlled fashion.
Examples of Globular Proteins
- Myoglobin: A protein that stores oxygen in muscle cells. It has a similar function to hemoglobin but is used in muscle tissues for oxygen storage and release during muscle contraction.
- Catalase: An enzyme that catalyzes the decomposition of hydrogen peroxide into water and oxygen, protecting cells from oxidative damage.
- Immunoglobulins (Antibodies): Proteins that bind to specific antigens to neutralize or mark them for destruction by the immune system.
- Albumin: The most abundant protein in blood plasma, it helps maintain osmotic pressure and transports various substances including hormones and drugs.
Fibrous Proteins
Fibrous proteins are a class of proteins characterized by their elongated, thread-like structures. Unlike globular proteins, fibrous proteins are generally insoluble in water and serve primarily structural roles in cells and tissues. Their unique structure provides strength and support to various biological components.
Characteristics
- Insolubility: Fibrous proteins are typically insoluble in water due to the presence of hydrophobic (water-repelling) amino acid residues that dominate their surface, making them less interactive with water molecules.
- Structure: They have a simple, repetitive primary and secondary structure, which leads to their extended and filamentous form. Key structural characteristics include:
- Primary Structure: A repetitive sequence of amino acids.
- Secondary Structure: They predominantly form alpha-helices or beta-sheets, which further assemble into supercoiled coils or stacked beta-sheets.
- Tertiary and Quaternary Structures: These structures are often simple or absent, focusing on the alignment and packing of the fibrous strands.
- Functionality: Fibrous proteins provide mechanical support, strength, and elasticity to tissues, playing a crucial role in maintaining the structural integrity of cells and organisms.
Functions of Fibrous Proteins
- Structural Support: They form the building blocks of connective tissues, bones, skin, hair, and nails. Their primary role is to provide strength, support, and elasticity.
- Protective Roles: Some fibrous proteins create protective barriers or coverings. For example, keratin forms the outer layer of human skin, providing protection against environmental damage.
- Mechanical Properties: They impart mechanical properties such as tensile strength and elasticity to various biological materials, enabling them to withstand physical stress.
Examples of Fibrous Proteins
- Collagen: The most abundant protein in the human body, collagen forms the structural framework of bones, tendons, ligaments, and skin. Its triple-helix structure provides high tensile strength.
- Keratin: Found in hair, nails, feathers, and the outer layer of skin, keratin provides protective and structural functions. It is highly durable and resistant to mechanical stress and water.
- Elastin: Present in connective tissues, elastin provides elasticity, allowing tissues like skin, lungs, and blood vessels to resume their shape after stretching or contracting.
- Fibroin: The primary protein in silk, fibroin contributes to the strength and flexibility of silk fibers, produced by spiders and silkworms.
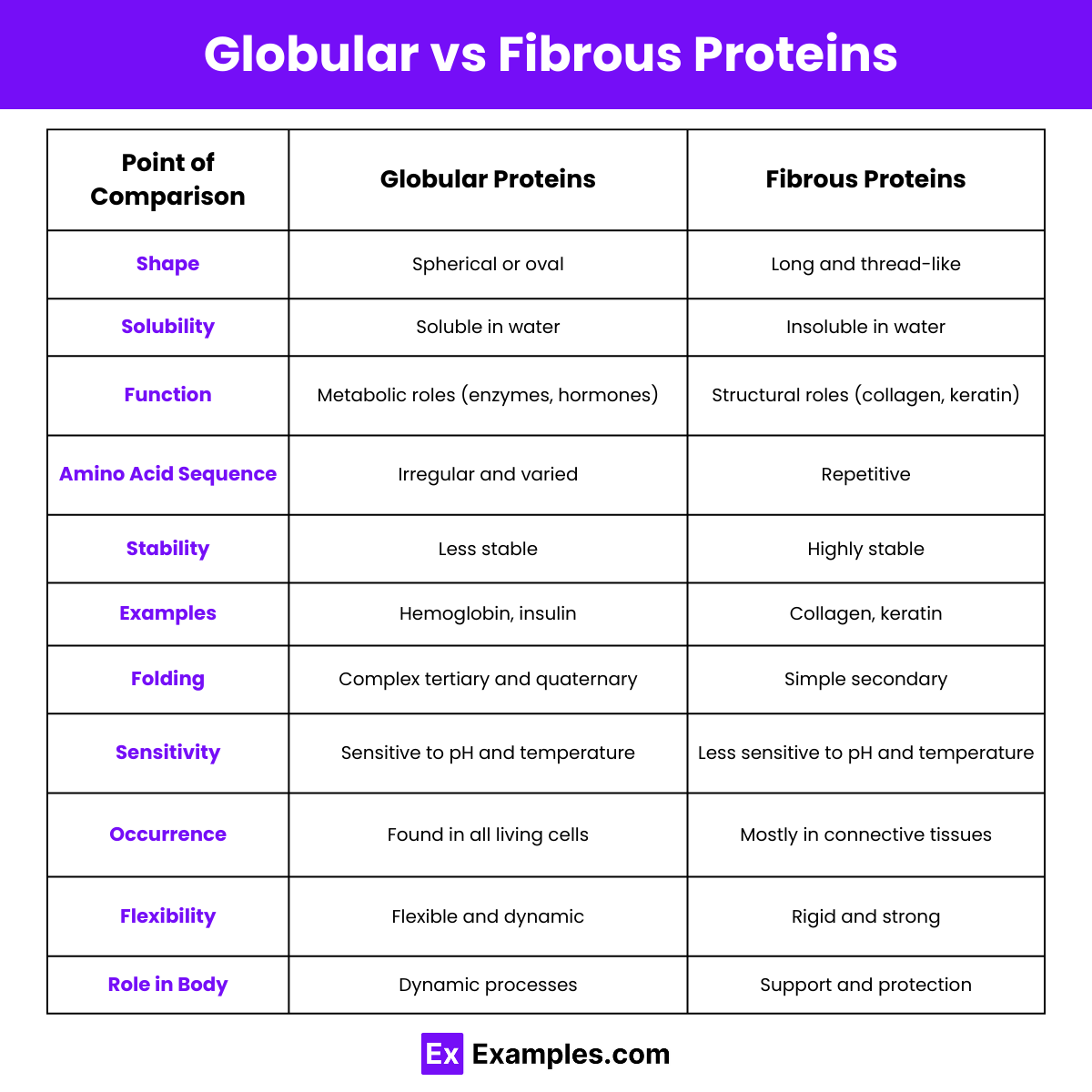
Difference Between Globular and Fibrous Proteins
| Point of Comparison | Globular Proteins | Fibrous Proteins |
|---|---|---|
| 1. Structure | Spherical or globular shape | Long, fiber-like shape |
| 2. Solubility | Soluble in water and other polar solvents | Insoluble in water and other polar solvents |
| 3. Function | Mainly involved in metabolic functions | Provide structural support and strength |
| 4. Example Proteins | Hemoglobin, enzymes, insulin | Collagen, keratin, elastin |
| 5. Amino Acid Sequence | Irregular amino acid sequence | Regular, repetitive amino acid sequence |
| 6. Stability | Less stable, sensitive to environmental changes | Highly stable, resistant to environmental changes |
| 7. Folding | Tightly folded into a compact shape | Extended and aligned in a parallel fashion |
| 8. Tertiary Structure | Well-defined tertiary structure | Primarily secondary structure, less tertiary structure |
| 9. Bonding | Held together by various types of bonds (hydrogen, ionic, etc.) | Held together mainly by hydrogen bonds |
| 10. Movement | Flexible, can undergo conformational changes | Rigid, limited flexibility |
| 11. Role in the Body | Enzymatic, regulatory, transport, and immune functions | Structural and protective roles |
| 12. Thermal Stability | Generally less thermally stable | More thermally stable |
| 13. Examples in Body | Myoglobin (muscle), immunoglobulins (immune system) | Collagen (connective tissue), keratin (hair and nails) |
| 14. Presence in Cells | Found throughout the cell, including cytoplasm and organelles | Found in extracellular matrix, tendons, and ligaments |
| 15. Crystallization | Can form crystals, often used in X-ray crystallography | Generally do not form crystals |
Similarities Between Globular and Fibrous Proteins
1. Basic Building Blocks
Both globular and fibrous proteins are composed of amino acids. These amino acids are linked together by peptide bonds to form polypeptide chains. The sequence of amino acids determines the protein’s structure and function.
2. Peptide Bonds and Polypeptide Chains
In both types of proteins, amino acids are connected through peptide bonds, forming long polypeptide chains. The peptide bond is a covalent bond that links the carboxyl group of one amino acid to the amino group of another.
3. Primary Structure
Both globular and fibrous proteins have a primary structure, which is the linear sequence of amino acids in the polypeptide chain. This sequence is determined by the genetic code and is critical for the protein’s final structure and function.
4. Role in Cellular Functions
Both globular and fibrous proteins play crucial roles in various cellular functions. They are involved in processes such as catalysis, transport, support, and defense. Their specific roles are determined by their structures, but both types are essential for maintaining cellular and organismal health.
5. Protein Folding
Both types of proteins undergo folding to achieve their functional conformations. The folding process is guided by the primary structure and involves various interactions, such as hydrogen bonds, ionic bonds, hydrophobic interactions, and disulfide bridges.
6. Importance of Structure-Function Relationship
For both globular and fibrous proteins, the relationship between structure and function is fundamental. The three-dimensional conformation of these proteins dictates their specific roles in the cell. Any alteration in their structure can lead to loss of function and potentially cause diseases.
7. Presence in All Living Organisms
Globular and fibrous proteins are found in all forms of life, from simple unicellular organisms to complex multicellular organisms. They are vital for various biological processes across different species.
8. Synthesis by Ribosomes
Both types of proteins are synthesized by ribosomes in the cell. The ribosomes translate the genetic information encoded in mRNA to assemble the amino acids in the correct sequence, forming the polypeptide chains that fold into functional proteins.
How do globular and fibrous proteins differ in structure?
Globular proteins are spherical and compact, while fibrous proteins are elongated and linear, forming fibers or sheets.
What is the solubility of globular proteins?
Globular proteins are generally soluble in water and aqueous solutions due to their hydrophilic outer surface.
What is the solubility of fibrous proteins?
Fibrous proteins are usually insoluble in water, providing structural stability and strength to tissues.
What is an example of a globular protein?
Hemoglobin, an oxygen-transporting protein in red blood cells, is a common example of a globular protein.
What is an example of a fibrous protein?
Collagen, a primary component of connective tissues like skin and cartilage, is a well-known fibrous protein.
What functions do globular proteins perform?
Globular proteins perform various functions, including catalyzing reactions (enzymes), regulating processes (hormones), and immune responses (antibodies).
What functions do fibrous proteins perform?
Fibrous proteins provide mechanical support and strength to cells and tissues, aiding in structure and movement.
Are globular proteins involved in metabolic processes?
Yes, globular proteins are crucial in metabolic processes, acting as enzymes and hormones to regulate biochemical reactions.
Are fibrous proteins involved in metabolic processes?
No, fibrous proteins primarily provide structural support and are not typically involved in metabolic processes.
Do globular proteins have a quaternary structure?
Many globular proteins have a quaternary structure, formed by the assembly of multiple polypeptide chains.