What happens to a cell placed in a hypertonic solution?
It swells
It shrinks
It remains the same size
It bursts

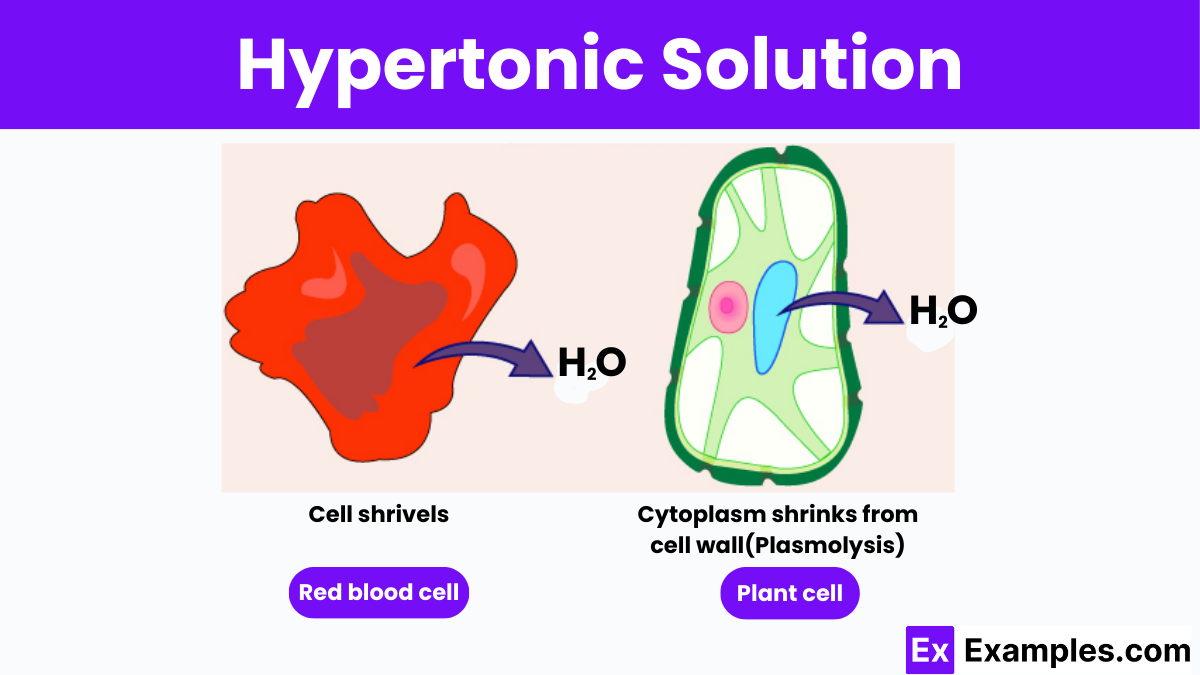
A hypertonic solution has a higher solute concentration and lower water concentration compared to body fluids. When cells are placed in such a solution, water moves out of the cells to equalize solute levels, a process known as osmosis. This causes cells to shrink and possibly undergo plasmolysis, where the cell membrane contracts and detaches from the cell wall, leading to potential cellular death
A hypertonic solution has a higher concentration of solutes compared to another solution across a semipermeable membrane. This type of solution exerts greater osmotic pressure and draws water out of cells placed in it, potentially leading to cell shrinkage or crenation.
In a hypertonic solution, water movement across a semipermeable membrane seeks to balance solute concentrations on both sides. When two solutions are isotonic, water flows equally in both directions, maintaining equilibrium. However, in a system where one side is hypertonic (higher solute concentration) and the other is hypotonic (lower solute concentration), water naturally flows from the hypotonic side to the hypertonic side. This osmotic flow continues until the solute concentrations equalize, resulting in isotonic conditions. This dynamic is crucial for understanding cellular processes and the physiological responses of organisms to their environments.
Red blood cells (RBCs) provide a classic example of how tonicity affects cells. In a hypertonic solution, where the solute concentration outside the cell is higher than inside, water moves out of the RBCs to balance the solute levels. This causes the cells to shrink and undergo crenation, resulting in a scalloped appearance that impacts their functionality. This phenomenon illustrates the critical importance of maintaining proper solute balance in medical treatments and biological research, as improper solute concentrations can severely affect cell health and functionality.
A hypertonic cell refers to a cell that is placed in a hypertonic solution, where the external environment has a higher concentration of solutes than the cell’s internal fluid. This imbalance causes water to flow out of the cell through osmosis in an effort to equalize the solute concentrations across the cell membrane. As a result, the cell loses water and shrinks, a process known as crenation in animal cells or plasmolysis in plant cells. This cellular response to a hypertonic environment is crucial for understanding fluid balance in biological systems and is widely studied in medical and biological research to understand cell behavior under stress conditions.
When plant cells are placed in a hypertonic solution, the external environment has a higher concentration of solutes than the fluid within the cells. This causes water to move out of the plant cells into the surrounding solution through osmosis, aiming to equalize the solute concentrations on both sides of the cell membrane. As the water exits, the cell’s cytoplasm shrinks and the plasma membrane pulls away from the cell wall in a process known as plasmolysis. This results in the plant cell becoming flaccid, which can lead to wilting in the tissues of living plants. This effect is critical to understanding how plants respond to drought and saline conditions, affecting their growth and survival.
When a cell is placed in a hypertonic solution, it experiences osmotic pressure that causes the cell to lose water to its environment. This process is critical to understand, especially in fields like biology, microbiology, and medicine, as it affects cellular function and health.
Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. In the context of a hypertonic solution, the outside environment has a higher solute concentration compared to the cell’s interior.
Water Loss and Cell Shrinkage:
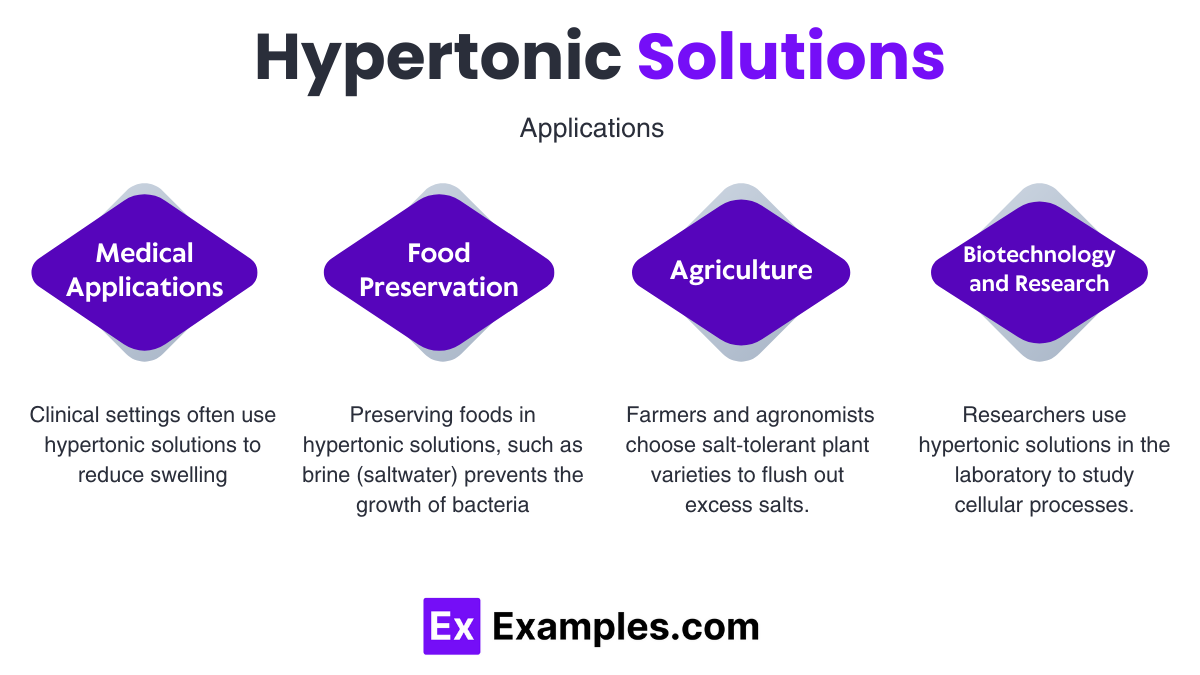
Treating Edema:
Hydration Control in Patients:
Increased Shelf Life:
Soil Salinity Management:
Rapid Rehydration Techniques:
Cellular Studies:
A hypertonic solution example is seawater, which has higher solute concentration than human blood cells.
Hypotonic solutions have fewer solutes compared to hypertonic solutions, which have more.
An isotonic solution has equal solute concentration as the cells, while a hypertonic solution has more.
A hypotonic solution has lower solute concentration compared to the inside of a cell, causing cells to swell.
Hypertonic solutions cause cells to shrink as water moves out to balance solute concentrations.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What happens to a cell placed in a hypertonic solution?
It swells
It shrinks
It remains the same size
It bursts
Which of the following best describes a hypertonic solution?
Lower solute concentration than the cell
No solute concentration
Higher solute concentration than the cell
Equal solute concentration as the cell
What is the primary movement of water when a cell is placed in a hypertonic solution?
Into the cell
Random movement of water
No movement of water
Out of the cell
Which of the following solutions is an example of a hypertonic solution?
Distilled water
0.9% saline solution
10% saline solution
Pure alcohol
In a hypertonic environment, what will happen to a plant cell?
It will undergo plasmolysis
It will remain unchanged
It will burst
It will become turgid
Which of the following best describes the effect of a hypertonic solution on red blood cells?
Hemolysis
Crenation
Swelling
No effect
What is the result of placing an animal cell in a hypertonic solution?
Lysis
Equilibrium
Dehydration
Active transport
Why do hypertonic solutions cause cells to lose water?
Higher external solute concentration
Lower external solute concentration
Equal solute concentration
No solute concentration
Which of the following processes occurs when a cell is placed in a hypertonic solution?
Endocytosis
Exocytosis
Osmosis
Active transport
Which of the following would you expect in a hypertonic intravenous (IV) solution?
It will rehydrate cells
It will draw water out of cells
It will have no effect on cells
It will dilute the blood
Before you leave, take our quick quiz to enhance your learning!

