What is the atomic number of mendelevium?
101
100
102
99
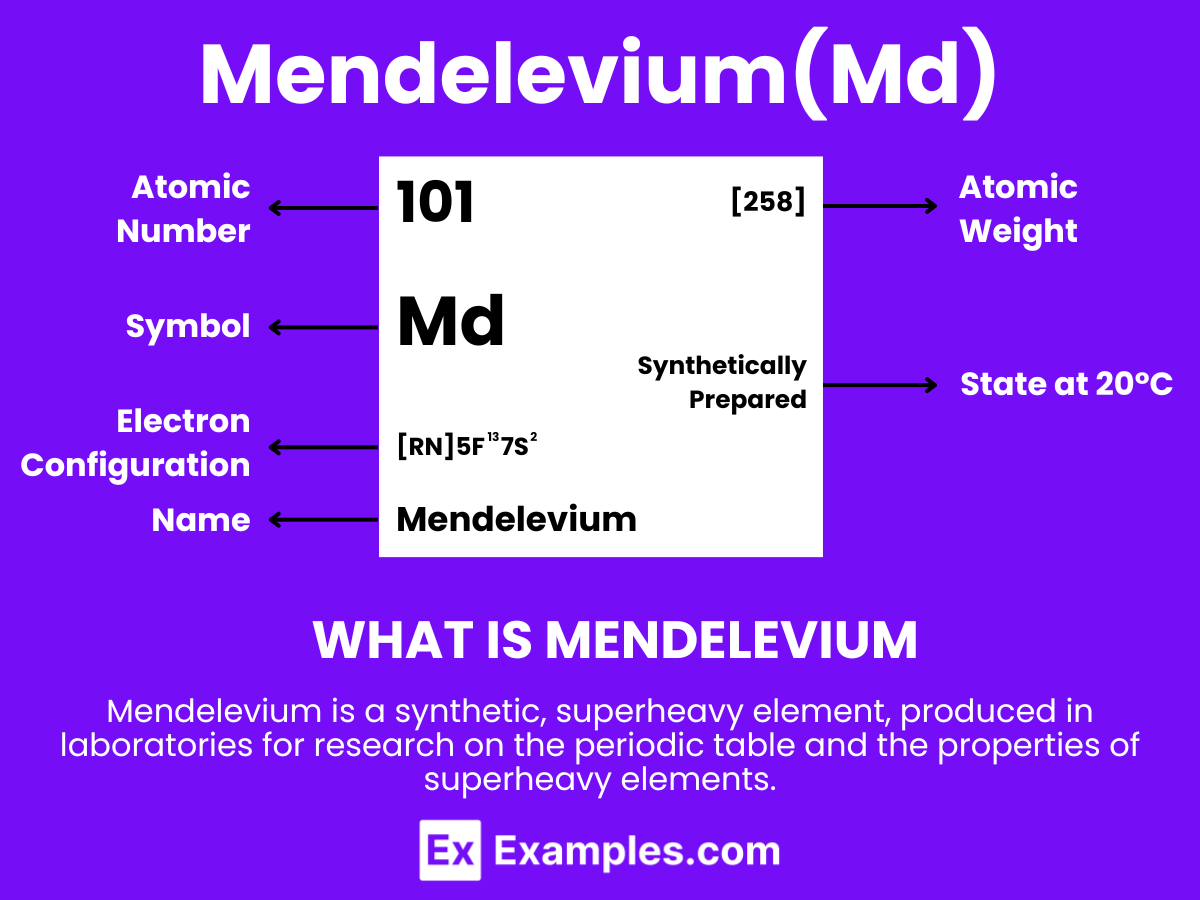
“Mendelevium” can be intriguing. Let’s dive into the fascinating world of Mendelevium, a synthetic element with the symbol Md and atomic number 101. Discovered in the midst of the 20th century, this element marks a significant milestone in the realm of nuclear science and chemistry. Named after the legendary chemist Dmitri Mendeleev, Mendelevium embodies the relentless pursuit of scientific discovery and innovation. With its unique properties and limited applications, Mendelevium sparks curiosity among scientists and enthusiasts alike. As we explore its characteristics, synthesis, and role in advancing the periodic table’s understanding, we uncover the marvels of this elusive element. This brief glimpse into Mendelevium not only enriches our knowledge but also highlights the element’s contribution to the ever-evolving field of science.
Mendelevium is a synthetic element with the chemical symbol Md and atomic number 101. It is known for being produced in particle accelerators through the bombardment of atomic nuclei. Mendelevium does not occur in nature and has a very short lifespan before it decays, which presents challenges for its study. The element’s discovery is crucial for nuclear physics research, especially in exploring the properties and behaviors of transuranium elements in the periodic table. Because of its significant instability and radioactivity, mendelevium has no practical applications beyond scientific inquiry, where it contributes to our understanding of the chemical characteristics of heavy elements and the limits of the periodic table.
Understanding the atomic structure of Mendelevium provides valuable insights into its unique position within the periodic table and nuclear chemistry. With 101 protons in its nucleus, Mendelevium’s atomic identity is firmly established, yet its chemical properties and potential for molecular formation remain largely enigmatic.
Atomic Level: Each atom of Mendelevium (Md) is characterized by having 101 protons in its nucleus, defining its atomic number as 101. The theoretical electron configuration of Mendelevium is [Rn]5f¹³ 7s², indicating it has a nearly complete 5f orbital, with two electrons in its 7s orbital, laying the groundwork for chemical interactions. However, relativistic effects are expected to significantly influence its actual electron configuration, potentially altering its chemical properties.
Molecular Formation: Unlike simpler elements that can form diatomic molecules (such as H₂), Mendelevium does not naturally form molecules or exhibit a stable molecular structure due to its extremely short half-life and high instability. The element exists for only moments before decaying into lighter elements, making the study of its bonding characteristics and molecular formation largely theoretical. In the hypothetical scenario where Mendelevium atoms could persist long enough to interact chemically, their behavior would likely be influenced by their electron configuration, but this remains speculative.
The stability and phase of Mendelevium under various temperatures and pressures are subjects of theoretical speculation, as its brief existence precludes the observation of solid, liquid, or gaseous states under normal conditions. The term “Mendelevium Gas” does not apply in the same way it might for simpler elements or compounds, given its complex and highly unstable nature in the context of nuclear chemistry.
| Property | Value |
|---|---|
| Appearance | Unknown; presumably metallic |
| Atomic Number | 101 |
| Atomic Mass | (258)amu |
| State at 20 °C | Solid (predicted) |
| Melting Point | 827 °C (predicted) |
| Boiling Point | Not precisely known; estimated to be around 800 °C (prediction based on extrapolation from lighter elements) |
| Density | Estimated to be around 10.3 g/cm³ (predicted) |
| Electron Configuration | [Rn] 5f¹³ 7s² (predicted) |
| Oxidation States | +2, +3 (most stable) |
| Crystal Structure | Face-centered cubic (predicted) |
| Electronegativity | Pauling scale: 1.3 (estimated) |
| Ionization Energies | First: 635.9 kJ/mol (estimated) |
| Thermal Conductivity | Not determined |
| Magnetic Ordering | Not determined |
| Property | Value |
|---|---|
| Half-lives | Varies by isotope; from milliseconds to hours |
| Decay Modes | Alpha decay (most common), spontaneous fission |
| Neutron Cross Section | Not well characterized |
| Neutron Mass Absorption | Not determined |
| Isotopes | Over 15, with ^256Md and ^258Md being among the most stable |
Target Material
A compound illustrating the reaction between mendelevium and oxygen to form an oxide.
Equation: Md+3O₂→2Md₂O₃
Predicts the formation of a fluoride compound when mendelevium reacts with fluorine.
Equation: Md+3F₂→MdF₃
Suggests the possibility of mendelevium combining with chlorine to form a chloride compound.
Equation: Md+3Cl₂→MdCl₃
Indicates the theoretical reaction between mendelevium and bromine to produce a bromide.
Equation: Md+3Br₂→MdBr₃
Inference about mendelevium’s ability to react with iodine to form an iodide compound.
Equation: Md+3I2→MdI₃
Speculates on the reaction between mendelevium and hydrogen to create a hydride.
Equation: Md+3H2→2MdH3
| Isotope | Half-Life | Decay Mode(s) | Daughter Isotope(s) |
|---|---|---|---|
| ^245Md | 0.9 seconds | Electron capture, Alpha decay | ^245Fm, ^241Es |
| ^246Md | 1.1 seconds | Alpha decay | ^242Es |
| ^247Md | 1.12 seconds | Alpha decay | ^243Es |
| ^248Md | 7 seconds | Alpha decay | ^244Es |
| ^249Md | 24 seconds | Alpha decay | ^245Es |
| ^250Md | 52 seconds | Alpha decay | ^246Es |
| ^251Md | 4 minutes | Alpha decay | ^247Es |
| ^252Md | 2.3 minutes | Alpha decay | ^248Es |
| ^253Md | 12 minutes | Alpha decay | ^249Es |
| ^254Md | 10 minutes | Alpha decay | ^250Es |
| ^255Md | 27 minutes | Alpha decay | ^251Es |
| ^256Md | 77 minutes | Alpha decay, Spontaneous fission | ^252Es, Fission Products |
| ^257Md | 5.52 hours | Alpha decay | ^253Es |
| ^258Md | 51.5 days | Alpha decay, Electron capture, Spontaneous fission | ^254Es, ^258Fm, Fission Products |
| ^259Md | 1.6 hours | Alpha decay, Electron capture | ^255Es, ^259Fm |
| ^260Md | 27.8 days | Spontaneous fission, Alpha decay | Fission Products, ^256Es |
Mendelevium is a synthetic and highly radioactive element with very limited applications due to its scarcity, short half-life, and the challenges associated with producing it. However, its study has contributed to various scientific fields. Here are some of the uses and contributions of mendelevium:
Mendelevium, with its atomic number of 101, is one of the synthetic elements in the actinide series that has captivated scientists since its discovery.
This article provided an in-depth exploration of mendelevium, covering its physical, thermodynamic, material, electromagnetic, and nuclear properties, alongside its known isotopes. Despite the challenges posed by its radioactivity and scarcity, mendelevium offers invaluable insights into the behavior and characteristics of transuranium elements, contributing significantly to scientific knowledge in nuclear chemistry and physics.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of mendelevium?
101
100
102
99
Mendelevium was named after which scientist?
Dmitri Mendeleev
Marie Curie
Albert Einstein
Niels Bohr
Which category of elements does mendelevium belong to?
Lanthanides
Actinides
Transition metals
Noble gases
What is the chemical symbol for mendelevium?
Md
Me
Mdium
Mn
In which year was mendelevium first synthesized?
1949
1955
1963
1972
What type of decay is most commonly observed in mendelevium isotopes?
Positron emission
Beta decay
Gamma decay
Alpha decay
Which method was used to first produce mendelevium?
Neutron capture
Nuclear fission
Proton bombardment
Electron capture
What is the primary use of mendelevium in scientific research?
Medicine
Nuclear reactors
Basic scientific research
Industrial applications
Mendelevium belongs to which block of the periodic table?
s-block
p-block
d-block
f-block
Which of the following properties is most notable for mendelevium?
High stability
High reactivity
High density
Radioactivity
Before you leave, take our quick quiz to enhance your learning!

