What is radiation?
The transfer of heat through a liquid
The transfer of heat through direct contact
The transfer of energy through space or a medium
The transfer of energy through a solid
Radiation, a fundamental concept in physics, encompasses a range of phenomena from sunlight to radio waves. This guide provides a thorough overview, presenting radiation in a clear, understandable manner. With practical examples, it demystifies radiation, making it an accessible and engaging topic for educators and students. Whether it’s understanding cosmic rays or the mechanics of microwaves, this guide illuminates the diverse aspects and applications of radiation.
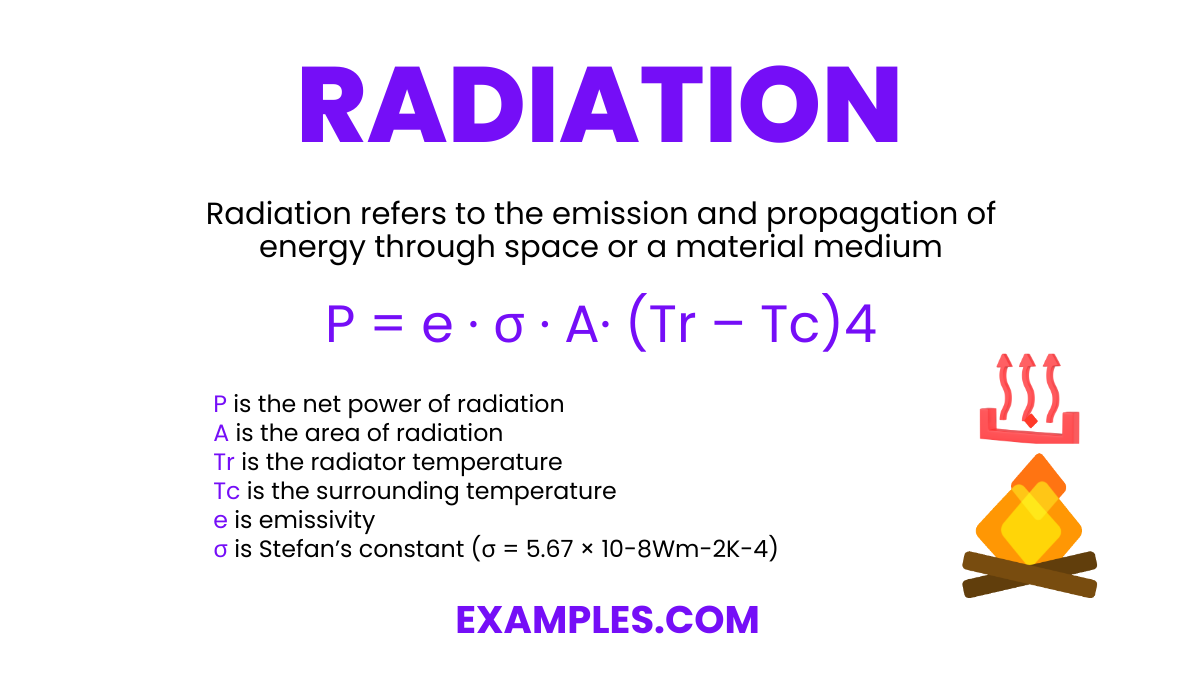
Radiation refers to the emission and propagation of energy through space or a material medium. This includes electromagnetic radiation like light and radio waves, and particle radiation such as alpha and beta radiation. Understanding radiation is key in fields ranging from astronomy to medicine, as it plays a vital role in various technologies and natural processes.
 A prime example of radiation is sunlight. The sun radiates energy in the form of light and heat, reaching Earth through the vacuum of space. This solar radiation is crucial for life, driving weather patterns and providing energy. It’s a clear, everyday example of electromagnetic radiation, essential for understanding broader concepts in physics and environmental science.
A prime example of radiation is sunlight. The sun radiates energy in the form of light and heat, reaching Earth through the vacuum of space. This solar radiation is crucial for life, driving weather patterns and providing energy. It’s a clear, everyday example of electromagnetic radiation, essential for understanding broader concepts in physics and environmental science.
The radiation formula calculates the energy radiated per unit area (E) by a black body in terms of its absolute temperature (T) and the Stefan-Boltzmann constant (σ). It is expressed as:
P = e ∙ σ ∙ A· (Tr – Tc)4
Where,
The unit used to measure radiation heat transfer is the “Watt per square meter per Kelvin” (W/(m²·K)).
Radiation, a broad and multifaceted concept, manifests in various forms across numerous applications. This collection of 22 examples provides a comprehensive view of radiation’s presence in both natural environments and technological applications. Each example is chosen for its clarity and relevance, aiding educators in illustrating the diverse roles and types of radiation. From everyday household devices to complex cosmic phenomena, these instances offer a well-rounded understanding of radiation’s extensive reach and significance.
Radiation is not just a scientific term; it’s a part of our daily experiences. This section sheds light on five examples of radiation in everyday life, illustrating its practical implications and presence around us. From household chores to leisure activities, these examples highlight how radiation, in various forms, plays a role in common daily tasks and amenities. These instances help educators explain the concept of radiation in a relatable and understandable manner.
Radiation heat transfer is a crucial aspect of cooking, influencing the taste and texture of food. This section offers five examples of how radiation heat transfer is used in various cooking methods. Understanding these examples provides insight into the practical application of radiation in culinary processes, making it a relevant topic for educators in explaining heat transfer concepts.
Radiation, a fundamental physical phenomenon, comes in various types, each with unique characteristics and applications. Broadly, it can be categorized into Electromagnetic Radiation and Particle Radiation. Electromagnetic Radiation includes visible light, radio waves, X-rays, etc., and is essential in communication and medical imaging. Particle Radiation involves particles like alpha and beta particles, significant in nuclear reactions and research. Understanding these types helps in grasping radiation’s role in technology, medicine, and natural processes.
Ionizing and Non-Ionizing Radiations differ mainly in their energy levels and interaction with matter. Ionizing Radiation has enough energy to remove tightly bound electrons from atoms, thus ionizing them. Non-Ionizing Radiation lacks this energy and mainly causes excitation but not ionization.
In conclusion, radiation, in its myriad forms, is an integral aspect of our universe. From the medical marvels achieved through X-rays and gamma rays to the everyday convenience of microwaves and radio waves, its impact is profound. Understanding radiation’s types and effects, both beneficial and harmful, is crucial in harnessing its power responsibly and safeguarding against its risks.
Scientists have devised a formula that determines the rate of heat transfer of radiation in a given situation. The formula is Q/t=?eAT^4, where Q/t is the rate of heat transfer, ? is the Stefan-Boltzman constant (?=5.67×10?8J/s?m2?K4), A is the area of the object, and T^4 is the absolute temperature in Kelvin (K).
Begin by writing down and understanding the formula for radiation. This will help provide a salient outline and structure that you can use as a reference for the final equation.
You must list out the numbers given by the question so that you can easily pinpoint and substitute said numbers for the correct variables. Be sure to have the correct measurements required by the formula for radiation, if it isn’t correct you will need to convert said measurements.
You must now substitute the numbers with their associated variables in the formula to create the equation. Ensure that the missing variable is on the left-hand side of the equation.
After you have solved the radiation equation, you must list out the measurement of the answer on the right-hand side of the number. If the measurement required by the question is different, convert the answer accordingly.
Radiation is a naturally occurring aftereffect of specific materials that will cause damage to an organism’s DNA, which could cause unwanted mutations and cellular damage. The DNA contains instructions and information for specific cells that will allow the said cells to function or do a specific task. If the DNA gets damaged, the cells will not know how to function properly and will cause damage to the host that cannot be undone. Not only that but radiation will also stick to the organism and materials it has made contact with, which means that the organism or material will also be emitting specific levels of radiation. Radiation causes specific diseases like Cutaneous Radiation Injuries (CRI) and Acute Radiation Syndrome (ARS) All of these issues only occur when the organism is subject to heavy and dangerous levels of radiation, similar to the levels caused by a nuclear missile or an atomic bomb.
A person’s body is adversely affected by radiation, which can harm an organism’s DNA and set off a chain reaction. When given at high doses, radiation therapy disrupts cancer cells’ DNA, either killing them or limiting their ability to proliferate. Cancer cells with irreparable DNA damage either stop reproducing or pass away. The body destroys and gets rid of the harmed cells after they pass away. Cancer cells are not quickly destroyed by radiation therapy. Days or weeks of treatment are needed before the DNA damage is sufficient to kill cancer cells. Cancer cells continue to die for a few weeks or months after radiation therapy is done.
Radiation is an energy that can travel through space and has the same speed as light, which means that almost any object emits a specific amount of radiation. This means that radiation is everywhere, but the more common types of this energy have trouble penetrating through the materials of the object emitting them. Therefore it is important to know and understand the concept of radiation, how to interact with it, and keep one’s safety from radiation.
Text prompt
Add Tone
22 Radiation Examples
Radiation Examples In Everyday Life
What is radiation?
The transfer of heat through a liquid
The transfer of heat through direct contact
The transfer of energy through space or a medium
The transfer of energy through a solid
Which type of radiation has the shortest wavelength?
Radio waves
Infrared radiation
Ultraviolet radiation
Gamma rays
Which of the following is an example of ionizing radiation?
Microwaves
Visible light
X-rays
Radio waves
What is the primary source of natural background radiation?
Cosmic rays
Medical X-rays
Nuclear power plants
Radon gas
Which type of radiation is used in cancer treatment?
Ultraviolet radiation
Infrared radiation
Gamma radiation
Radio waves
Which type of radiation is most likely to penetrate human tissue?
Alpha particles
Beta particles
Gamma rays
Ultraviolet rays
What type of radiation is commonly used in household smoke detectors?
Beta radiation
Gamma radiation
Alpha radiation
Ultraviolet radiation
Which of the following best describes alpha particles?
High-energy photons
Helium nuclei
Electrons
Neutrons
Which type of radiation has the longest wavelength?
Gamma rays
Ultraviolet radiation
Infrared radiation
Radio waves
What is the effect of ionizing radiation on biological tissues?
It heats the tissues
It changes the color of tissues
It ionizes atoms and molecules, potentially causing damage
It has no effect
Before you leave, take our quick quiz to enhance your learning!

