What is thermal energy?
The energy stored in chemical bonds
The energy associated with the motion of particles in a substance
The energy stored in atomic nuclei
The energy from electromagnetic waves
Thermal energy, a fundamental concept in physics, is vital for understanding how heat affects matter. This comprehensive guide breaks down thermal energy into simple, digestible explanations, perfect for teachers and students. Explore real-world examples, learn about heat transfer methods, and discover the everyday significance of this invisible yet powerful energy. Ideal for educators, this guide transforms complex scientific ideas into engaging, easy-to-understand lessons.
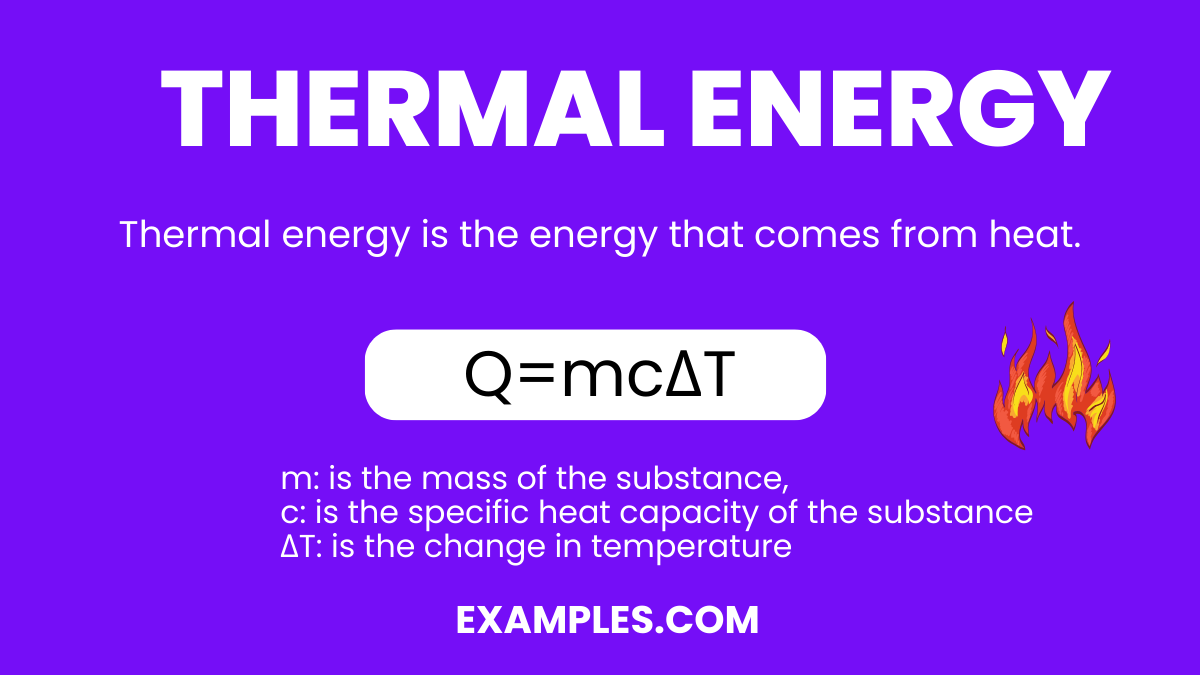
Thermal energy is the energy that comes from heat. This heat is generated by the movement of tiny particles within an object. The faster these particles move, the more heat they generate and the higher the thermal energy. In simple terms, thermal energy is what we feel as heat, whether from the sun on a summer day or from a warm stove. It’s a fundamental concept in physics, playing a crucial role in everything from weather patterns to the technology we use every day.
A classic example of thermal energy is boiling water. When water in a pot is heated on a stove, the heat from the stove increases the energy of the water molecules. These molecules move faster, causing the water temperature to rise until it reaches boiling point. This process demonstrates how heat energy is transferred to an object (water) and changes its physical state, from liquid to gas, showcasing the practical and observable effects of thermal energy.
The thermal energy (Q) of a substance can be calculated using the formula:
Where:
m: is the mass of the substance,
c: is the specific heat capacity of the substance (the amount of energy required to raise the temperature of one kilogram of the substance by one degree Celsius),
ΔT: is the change in temperature (in Celsius or Kelvin).
Thermal energy is a key concept in physics and everyday life. These 22 examples are designed to aid teachers in explaining this concept in a simple, engaging manner. They encompass a range of scenarios where thermal energy plays a vital role, aiding students in grasping its practical applications and improving their English vocabulary related to science.
Convection is the transfer of heat through fluids (liquids or gases) caused by molecular motion. In convection, warmer parts of a liquid or gas rise to cooler areas, and cooler liquid or gas moves down.
Example: Boiling water demonstrates convection, where heated water rises to the surface, cools, and then sinks back down.
Conduction is the process of heat transfer through direct contact of materials. It occurs when molecules in a substance, typically a solid, vibrate and pass their energy to neighboring molecules.
Example: A metal spoon getting hot from its handle down to its tip when left in a pot of boiling water.
Heat transfer is the movement of thermal energy from one object or substance to another. It can occur through conduction, convection, or radiation.
Example: Heat moving from a hot cup of coffee to the air, gradually cooling the coffee.
Radiation is the transfer of energy through electromagnetic waves. It does not require a medium to travel, meaning it can occur even through a vacuum.
Example: The heat from the sun reaching the Earth through space.
Thermal energy transfer refers to the movement of thermal energy from one object or system to another. It can be achieved through the methods of conduction, convection, and radiation.
Example: In a toaster, electrical energy is converted to thermal energy, which is transferred to the bread to toast it.
Geothermal energy is the heat derived from the Earth’s internal heat. It is a sustainable and clean source of energy.
Example: Iceland’s use of geothermal energy for heating homes and generating electricity.
Solar energy involves harnessing the thermal energy from the sun’s rays. It’s an abundant, renewable energy source.
Example: Solar panels converting sunlight into electricity for homes and businesses.
Fuel cell energy is generated through a chemical reaction, often using hydrogen. It’s efficient and produces minimal waste.
Example: Hydrogen fuel cells used in electric vehicles to produce power.
Melting ice is a process where solid water (ice) absorbs thermal energy and turns into liquid water.
Example: Ice cubes melting in a glass of water on a warm day.
This process uses temperature differences between the ocean’s surface and deeper layers to generate electricity.
Example: Power plants in tropical regions using warm surface water and cold deep water to produce power.
Thermal batteries store heat when available and release it when needed. They can store solar energy or excess heat.
Example: A solar thermal battery in a greenhouse, storing heat during the day and releasing it at night.
Thermal energy, a central concept in physics and daily life, plays multiple roles. It’s the driving force behind many processes, both natural and man-made.
Understanding the sources of thermal energy helps in appreciating its ubiquitous nature and applications.
Thermal energy plays a pivotal role in changing the states of matter – solid, liquid, and gas. This energy affects the movement of particles within a substance, leading to changes in its state. A higher thermal energy typically means particles move faster and farther apart. This concept is essential in understanding physical transformations in nature and science.
| Renewable Energy | Thermal Energy |
|---|---|
| Comes from natural resources like wind, solar, and water. | Refers to the energy possessed by an object due to the movement of its particles. |
| Renewable and can be replenished. | Not necessarily renewable; depends on the source. |
| Used in generating electricity and powering vehicles. | Used in heating, cooling, and mechanical work. |
| Has minimal environmental impact. | Can have varied environmental impacts depending on the source. |
| Examples include solar panels and wind turbines. | Examples include geothermal heating and steam engines. |
Dealing with thermal energy requires strict safety measures to prevent accidents and injuries. Understanding and implementing these precautions is crucial, especially in educational settings where demonstrations often occur.
src: content.schoolinsites.com
Download This Imagesrc: spsphysicalscience.pbworks.com
Download This Imagesrc: worldbook.com.au
Download This ImageIn conclusion, thermal energy is a fundamental concept in physics, influencing the states of matter and prevalent in everyday phenomena. Understanding its principles and safety measures is crucial, especially in educational settings. This guide aims to provide a clear, concise understanding of thermal energy, enhancing teaching and learning experiences.
Text prompt
Add Tone
22 Thermal Energy Examples
Thermal Energy Examples in Daily Life
What is thermal energy?
The energy stored in chemical bonds
The energy associated with the motion of particles in a substance
The energy stored in atomic nuclei
The energy from electromagnetic waves
Which of the following units is used to measure thermal energy?
Joules
Newtons
Pascals
Amperes
Which process involves the transfer of thermal energy by the movement of fluid?
Conduction
Convection
Radiation
Insulation
What is the primary mechanism of thermal energy transfer in a metal rod?
Conduction
Convection
Radiation
Reflection
Which of the following is an example of thermal energy transfer by radiation?
Boiling water
Heat from a campfire
Heating a metal rod in a flame
Warm air rising in a room
What happens to the thermal energy of an object when its temperature increases?
It decreases
It remains the same
It increases
It depends on the object’s mass
Which law states that energy cannot be created or destroyed, only transferred or converted?
Newton's First Law
Law of Conservation of Energy
Law of Thermodynamics
Coulomb's Law
In which state of matter does conduction occur most effectively?
Solid
Liquid
Gas
Plasma
What is specific heat capacity?
The total energy of an object
The amount of heat energy required to raise the temperature of 1 kg of a substance by 1°C
The energy required to change a substance from solid to liquid
The thermal energy transferred per unit time
Which material would make the best thermal insulator?
Copper
Aluminum
Water
Wool
Before you leave, take our quick quiz to enhance your learning!

