What is the atomic number of Iron?
24
26
28
30
Dive into the versatile world of iron, a cornerstone of modern civilization and industrial advancement. This comprehensive guide illuminates iron’s fundamental role in construction, manufacturing, and technology. With detailed examples, we explore its myriad uses, from the robust frameworks of skyscrapers to the critical components in electronic devices. Unravel the secrets of iron compounds, their applications in various industries, and how this abundant metal continues to shape our world. Discover iron’s impact, one innovation at a time.
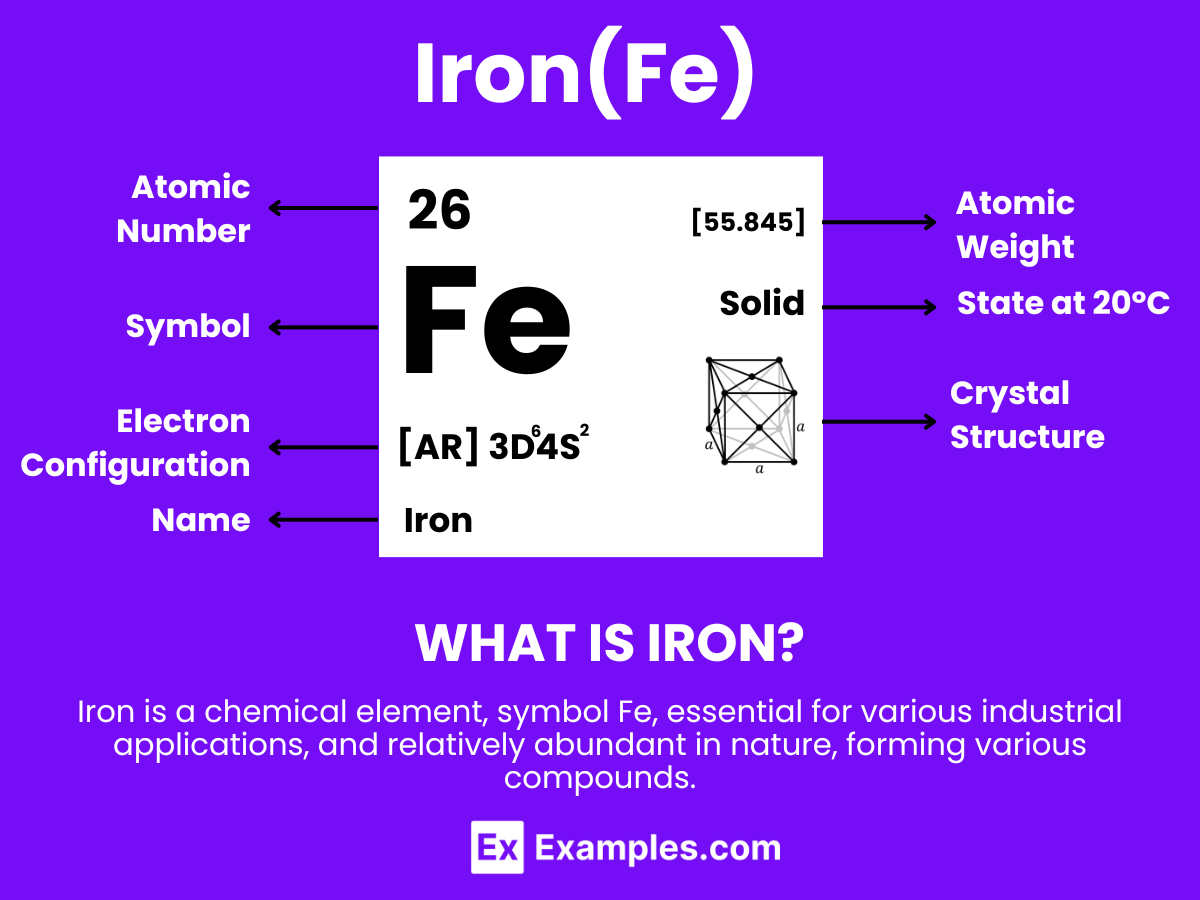
Iron is a chemical element with the symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal in the first transition series. Iron is the most common element on Earth by mass, forming much of Earth’s outer and inner core, and it is the fourth most common element in the Earth’s crust. Iron’s very common presence in the universe is due to its easily fused nucleus by moderate temperatures in stars formed from the collapse of giant gas clouds.
Iron is renowned for its magnetic properties, high malleability, ductility, and its ability to form various alloys with many other metals. Alloys of iron, such as steel, are crucial in modern engineering and construction because of their strength and durability. Iron also plays an essential role in biology, forming complexes with molecular oxygen in hemoglobin and myoglobin; these iron-oxygen complexes are what allow animals to metabolize oxygen for use in the body.
Formula: Fe
Composition: Consists of a single iron atom.
Bond Type: In its elemental form, iron does not form bonds as it is a pure element. However, iron can form both covalent and ionic bonds when reacting with other elements.
Molecular Structure: As a pure element, iron does not form a molecular structure in the same way compounds like H₂O do. At room temperature, iron exhibits a metallic state with a body-centered cubic (bcc) crystalline structure, transitioning to a face-centered cubic (fcc) structure at higher temperatures.
Electron Sharing: In compounds, iron typically engages in covalent sharing of electrons or transfers electrons ionically, depending on the nature of the other element(s) it bonds with.
Significance: Iron is known for its magnetic properties, structural strength, and versatility, making it foundational in construction, manufacturing, and transportation. Its role in the formation of hemoglobin in blood highlights its biological importance, facilitating oxygen transport in living organisms.
Role in Chemistry: Iron’s significant role spans from catalysis in industrial processes, such as the Haber-Bosch process for ammonia synthesis, to its function in organic chemistry and biochemistry. Iron compounds are pivotal in manufacturing, environmental remediation, and medical applications, underscoring its crucial position in both traditional and innovative technological advancements.
Iron, symbolized as Fe, holds the atomic number 26 in the periodic table, marking its significance in structural and biological realms.
| Property | Value |
|---|---|
| Atomic Number | 26 |
| Atomic Mass | 55.845 u |
| Density | 7.874 g/cm³ (at room temperature) |
| Melting Point | 1538°C (2800°F) |
| Boiling Point | 2862°C (5182°F) |
| Heat of Fusion | 13.81 kJ/mol |
| Heat of Vaporization | 340 kJ/mol |
| Specific Heat Capacity | 0.449 J/g·K (at 25°C) |
| Thermal Conductivity | 80.4 W/(m·K) |
| Electrical Resistivity | 9.71 µΩ·m (at 20°C) |
| Crystal Structure | Body-Centered Cubic (BCC) at room temperature, transforming to Face-Centered Cubic (FCC) at 912°C |
| Property | Value |
|---|---|
| Melting Point | 1538°C (2800°F) |
| Boiling Point | 2862°C (5182°F) |
| Heat of Fusion | 13.81 kJ/mol |
| Heat of Vaporization | 340 kJ/mol |
| Specific Heat Capacity | 0.449 J/g·K (at 25°C) |
| Thermal Conductivity | 80.4 W/m·K (at 25°C) |
| Property | Value |
|---|---|
| Density | 7.874 g/cm³ (at 20°C) |
| Young’s Modulus | 211 GPa |
| Tensile Strength | 370–540 MPa |
| Hardness (Brinell) | 200 HB (Pure iron) |
| Poisson’s Ratio | 0.29 |
| Elastic Modulus | 211 GPa |
| Property | Value |
|---|---|
| Electrical Resistivity | 96.1 nΩ·m (at 20°C) |
| Magnetic Susceptibility | High (Ferromagnetic) |
| Curie Temperature | 770°C (1418°F) |
| Relative Permeability | Around 5000 (for pure iron) |
| Coercivity | Approximately 0.1-1 A/m (for soft iron) |
| Property | Value |
|---|---|
| Atomic Number | 26 |
| Isotopes | Iron-54, Iron-56, Iron-57, Iron-58 |
| Natural Abundance | Iron-56: 91.75%, Iron-54: 5.85%, Iron-57: 2.12%, Iron-58: 0.28% |
| Half-Life | Stable (Iron-54, Iron-56, Iron-57), Iron-58: 44.503 days (radioactive) |
| Neutron Cross Section | Iron-56: 2.59 barns |
| Nuclear Spin | Iron-57: 1/2 |
The preparation of iron predominantly involves the extraction and purification of iron ore through various processes. The primary method is the blast furnace process, also known as the reduction of iron ore in a blast furnace. This process entails several steps to convert raw iron ore into usable iron.
Iron ore, containing iron oxides, is extracted from the earth. Common iron ores include hematite (Fe₂O₃), magnetite (Fe₃O₄), and limonite (FeO(OH)·nH₂O).
The extracted iron ore is crushed and then either washed to remove impurities or directly sent to the blast furnace. Limestone (calcium carbonate) and coke (a form of carbon) are also prepared as part of the raw materials.
The prepared iron ore, along with limestone and coke, is loaded into the top of the blast furnace. The furnace operates at high temperatures, typically around 2000°C.
The molten iron, which settles at the bottom of the furnace, is periodically tapped off. The lighter slag, which forms from the reaction of lime and silica, floats on top of the iron and is also removed.
The tapped molten iron can be directly used in steel-making processes or further processed into various forms of iron, including cast iron and wrought iron, through additional refining steps.
| Isotope | Mass Number | Half-Life | Notes |
|---|---|---|---|
| Fe-54 | 54 | Stable | Naturally occurring; 5.8% abundance |
| Fe-56 | 56 | Stable | Most abundant isotope; ~91.75% abundance |
| Fe-57 | 57 | Stable | Naturally occurring; 2.12% abundance |
| Fe-58 | 58 | Stable | Naturally occurring; 0.28% abundance |
| Fe-59 | 59 | 44.503 days | Radioactive; used in medical and research applications |
| Fe-60 | 60 | 2.6 million years | Radioactive; cosmogenic and used in geochronology |
Iron is one of the most extensively used metals in the world, owing to its abundance, durability, and versatility. Its applications span across various industries, reflecting its significance in our daily lives and global economy.
The production of iron primarily involves the extraction and processing of iron ore, a process that includes several key steps:
This traditional blast furnace method, known as the primary route, has been complemented by the direct reduction process, which produces solid iron from ore without melting, offering an energy-efficient alternative.
Iron, owing to its abundance and remarkable properties, finds applications across various sectors:
This article has explored the multifaceted nature of iron, delving into its various isotopes, widespread applications, essential physical and chemical properties, and the intricate process of its preparation. Iron’s significance in industrial, technological, and biological contexts underscores its irreplaceable role in modern society and highlights the ongoing need for understanding and innovating with this fundamental element.

Dive into the versatile world of iron, a cornerstone of modern civilization and industrial advancement. This comprehensive guide illuminates iron’s fundamental role in construction, manufacturing, and technology. With detailed examples, we explore its myriad uses, from the robust frameworks of skyscrapers to the critical components in electronic devices. Unravel the secrets of iron compounds, their applications in various industries, and how this abundant metal continues to shape our world. Discover iron’s impact, one innovation at a time.
Iron is a chemical element with the symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal in the first transition series. Iron is the most common element on Earth by mass, forming much of Earth’s outer and inner core, and it is the fourth most common element in the Earth’s crust. Iron’s very common presence in the universe is due to its easily fused nucleus by moderate temperatures in stars formed from the collapse of giant gas clouds.
Iron is renowned for its magnetic properties, high malleability, ductility, and its ability to form various alloys with many other metals. Alloys of iron, such as steel, are crucial in modern engineering and construction because of their strength and durability. Iron also plays an essential role in biology, forming complexes with molecular oxygen in hemoglobin and myoglobin; these iron-oxygen complexes are what allow animals to metabolize oxygen for use in the body.
Formula: Fe
Composition: Consists of a single iron atom.
Bond Type: In its elemental form, iron does not form bonds as it is a pure element. However, iron can form both covalent and ionic bonds when reacting with other elements.
Molecular Structure: As a pure element, iron does not form a molecular structure in the same way compounds like H₂O do. At room temperature, iron exhibits a metallic state with a body-centered cubic (bcc) crystalline structure, transitioning to a face-centered cubic (fcc) structure at higher temperatures.
Electron Sharing: In compounds, iron typically engages in covalent sharing of electrons or transfers electrons ionically, depending on the nature of the other element(s) it bonds with.
Significance: Iron is known for its magnetic properties, structural strength, and versatility, making it foundational in construction, manufacturing, and transportation. Its role in the formation of hemoglobin in blood highlights its biological importance, facilitating oxygen transport in living organisms.
Role in Chemistry: Iron’s significant role spans from catalysis in industrial processes, such as the Haber-Bosch process for ammonia synthesis, to its function in organic chemistry and biochemistry. Iron compounds are pivotal in manufacturing, environmental remediation, and medical applications, underscoring its crucial position in both traditional and innovative technological advancements.
Iron, symbolized as Fe, holds the atomic number 26 in the periodic table, marking its significance in structural and biological realms.
The nucleus of iron consists of 26 protons.
It has a varying number of neutrons; the most common isotopes include:
Iron-56 with 26 neutrons.
Iron-54 with 28 neutrons.
Iron-57 with 31 neutrons.
Iron’s electrons are distributed across four shells in the configuration of 2, 8, 14, 2.
This specific arrangement facilitates iron’s multiple oxidation states, predominantly +2 and +3.
The ability to form various oxidation states enhances iron’s versatility in compounds and alloys.
Unpaired electrons in its outermost shell render iron paramagnetic, contributing to its magnetic attributes.
The atomic structure underlines iron’s reactivity and diverse chemical behavior.
Its properties are crucial for applications in construction, manufacturing, and biological systems, especially for oxygen transport and metabolism
Property | Value |
|---|---|
Atomic Number | 26 |
Atomic Mass | 55.845 u |
Density | 7.874 g/cm³ (at room temperature) |
Melting Point | 1538°C (2800°F) |
Boiling Point | 2862°C (5182°F) |
Heat of Fusion | 13.81 kJ/mol |
Heat of Vaporization | 340 kJ/mol |
Specific Heat Capacity | 0.449 J/g·K (at 25°C) |
Thermal Conductivity | 80.4 W/(m·K) |
Electrical Resistivity | 9.71 µΩ·m (at 20°C) |
Crystal Structure | Body-Centered Cubic (BCC) at room temperature, transforming to Face-Centered Cubic (FCC) at 912°C |
Oxidation States: Iron commonly exhibits two principal oxidation states: +2 (ferrous, Fe²⁺) and +3 (ferric, Fe³⁺). These states play a pivotal role in the formation of diverse compounds, ranging from simple oxides to complex coordination complexes.
Reaction with Oxygen: Iron reacts with oxygen in the presence of water or moisture to form rust, a process known as corrosion.
4Fe+3O₂+6H₂O→4Fe(OH)₃ This hydrated iron(III) oxide, under certain conditions, can further dehydrate to form Fe₂O₃·nH₂O, commonly known as rust.
Reaction with Halogens: Iron directly combines with halogens to form iron halides. For example, with chlorine, iron forms iron(III) chloride: 2Fe+3Cl₂→2FeCl₃
Reaction with Acids: Iron reacts with dilute hydrochloric acid to produce iron(II) chloride and hydrogen gas: Fe+2HCl→FeCl₂+H₂↑
With sulfuric acid, iron forms iron(II) sulfate and hydrogen: Fe+H₂SO₄→FeSO₄+H₂↑ However, concentrated nitric acid renders iron passive by forming a protective oxide layer, demonstrating its variable reactivity.
Thermal Decomposition: Iron(III) compounds, like iron(III) nitrate, decompose upon heating, producing iron(III) oxide, nitrogen dioxide, and oxygen: ↑2Fe(NO₃)₃→2Fe₂O₃+6NO₂+O₂↑
Catalytic Properties: Iron acts as a catalyst in various chemical reactions, including the Haber process for synthesizing ammonia from nitrogen and hydrogen: N2(g)+3H₂(g)Fe−catalyst→high pressure,2NH₃(g) This exemplifies iron’s vital role in industrial chemistry and its ability to lower reaction energy barriers.
Complex Formation: Iron forms complex ions, such as the hexaaquairon(II) ion,
[Fe(H2O)₆]₂⁺, and hexacyanoferrate(II), [Fe(CN)₆]₄⁻, showcasing its versatility in bonding and coordination chemistry.
Property | Value |
|---|---|
Melting Point | 1538°C (2800°F) |
Boiling Point | 2862°C (5182°F) |
Heat of Fusion | 13.81 kJ/mol |
Heat of Vaporization | 340 kJ/mol |
Specific Heat Capacity | 0.449 J/g·K (at 25°C) |
Thermal Conductivity | 80.4 W/m·K (at 25°C) |
Property | Value |
|---|---|
Density | 7.874 g/cm³ (at 20°C) |
Young’s Modulus | 211 GPa |
Tensile Strength | 370–540 MPa |
Hardness (Brinell) | 200 HB (Pure iron) |
Poisson’s Ratio | 0.29 |
Elastic Modulus | 211 GPa |
Property | Value |
|---|---|
Electrical Resistivity | 96.1 nΩ·m (at 20°C) |
Magnetic Susceptibility | High (Ferromagnetic) |
Curie Temperature | 770°C (1418°F) |
Relative Permeability | Around 5000 (for pure iron) |
Coercivity | Approximately 0.1-1 A/m (for soft iron) |
Property | Value |
|---|---|
Atomic Number | 26 |
Isotopes | Iron-54, Iron-56, Iron-57, Iron-58 |
Natural Abundance | Iron-56: 91.75%, Iron-54: 5.85%, Iron-57: 2.12%, Iron-58: 0.28% |
Half-Life | Stable (Iron-54, Iron-56, Iron-57), Iron-58: 44.503 days (radioactive) |
Neutron Cross Section | Iron-56: 2.59 barns |
Nuclear Spin | Iron-57: 1/2 |
The preparation of iron predominantly involves the extraction and purification of iron ore through various processes. The primary method is the blast furnace process, also known as the reduction of iron ore in a blast furnace. This process entails several steps to convert raw iron ore into usable iron.
Iron ore, containing iron oxides, is extracted from the earth. Common iron ores include hematite (Fe₂O₃), magnetite (Fe₃O₄), and limonite (FeO(OH)·nH₂O).
The extracted iron ore is crushed and then either washed to remove impurities or directly sent to the blast furnace. Limestone (calcium carbonate) and coke (a form of carbon) are also prepared as part of the raw materials.
The prepared iron ore, along with limestone and coke, is loaded into the top of the blast furnace. The furnace operates at high temperatures, typically around 2000°C.
a. The coke is burned to produce carbon monoxide (CO), which rises through the furnace.
b. The carbon monoxide acts as a reducing agent, converting the iron(III) oxide in the ore into molten iron: Fe2O₃+3CO→2Fe+3CO₂
c. Limestone is added to remove impurities. It decomposes to lime (CaO), which reacts with silica (SiO₂), an impurity in the ore, to form slag (calcium silicate):
CaCO₃→CaO+CO2
CaO+SiO₂→CaSiO₃
The molten iron, which settles at the bottom of the furnace, is periodically tapped off. The lighter slag, which forms from the reaction of lime and silica, floats on top of the iron and is also removed.
The tapped molten iron can be directly used in steel-making processes or further processed into various forms of iron, including cast iron and wrought iron, through additional refining steps.
Iron(II) Oxide (FeO) Iron(II) oxide is a black powder used in various industrial processes. Relevant
equation:2Fe+O₂→2FeO.
Iron(III) Oxide (Fe₂O₃) A reddish-brown compound, primarily used as a pigment and in rust prevention.
Equation: 4Fe+3O₂→2Fe₂O₃.
Iron(II,III) Oxide (Fe₃O₄) Also known as magnetite, this compound exhibits magnetic properties.
Equation:Fe+2Fe₂O₃→3Fe₃O₄.
Iron(II) Sulfide (FeS) A gray solid, used in the production of sulfuric acid.
Equation: Fe+S→FeS.
Iron(III) Chloride (FeCl₃) Used in water treatment and as an etching agent in circuit board production.
Equation: 2Fe+3Cl₂→2FeCl₃.
Iron(II) Sulfate (FeSO₄) Commonly used as a fertilizer and in the treatment of anemia. Equation: 2Fe+H₂SO₄→FeSO₄+H₂
Isotope | Mass Number | Half-Life | Notes |
|---|---|---|---|
Fe-54 | 54 | Stable | Naturally occurring; 5.8% abundance |
Fe-56 | 56 | Stable | Most abundant isotope; ~91.75% abundance |
Fe-57 | 57 | Stable | Naturally occurring; 2.12% abundance |
Fe-58 | 58 | Stable | Naturally occurring; 0.28% abundance |
Fe-59 | 59 | 44.503 days | Radioactive; used in medical and research applications |
Fe-60 | 60 | 2.6 million years | Radioactive; cosmogenic and used in geochronology |
Iron is one of the most extensively used metals in the world, owing to its abundance, durability, and versatility. Its applications span across various industries, reflecting its significance in our daily lives and global economy.
Steel Production: The primary use of iron is in the manufacturing of steel, an alloy of iron and carbon, and sometimes other elements. Steel’s strength and flexibility make it crucial in construction, automotive, and appliance industries.
Magnetic Materials: Due to its magnetic properties, iron is a key component in the manufacturing of magnets, which are used in motors, generators, data storage devices, and electrical transformers.
Catalysis: Iron serves as a catalyst in the Haber process for synthesizing ammonia, essential for fertilizers and therefore global agriculture.
Medicine: Iron is vital for human health, particularly in the transport of oxygen in the blood. Iron supplements are used to treat anemia and other iron deficiencies.
Pigments: Iron oxides are used as pigments in paints, coatings, and concrete products, offering colors from yellow to red and black.
Construction: Iron bars (rebar) reinforce concrete structures, providing strength and stability to buildings, bridges, and roads.
Chemical Industry: Iron compounds, like ferric chloride, are used in water and wastewater treatment processes as flocculants and coagulants.
Manufacturing: Iron is involved in the production of various goods, including machinery, tools, and hardware, due to its strength and workability
The production of iron primarily involves the extraction and processing of iron ore, a process that includes several key steps:
Mining: Iron ore is mined from the earth, with significant sources including hematite and magnetite ores.
Crushing and Grinding: The ore is crushed and ground to liberate the iron.
Concentration: Through processes such as magnetic separation, flotation, or gravity separation, the iron content is concentrated.
Reduction: The concentrated ore is then reduced in a blast furnace at high temperatures, using coke as a reducing agent.
Melting: Iron ore melts and separates from slag, producing molten iron.
Casting: The molten iron is poured into molds to form ingots or transferred to steelmaking processes.
This traditional blast furnace method, known as the primary route, has been complemented by the direct reduction process, which produces solid iron from ore without melting, offering an energy-efficient alternative.
Iron, owing to its abundance and remarkable properties, finds applications across various sectors:
Steel Production: The majority of iron is used to produce steel, an alloy that is fundamental to the construction and manufacturing industries.
Machinery and Automotive: Iron and its alloys are used in the fabrication of machinery, automotive components, and infrastructure.
Magnet Production: Due to its magnetic properties, iron is essential in the production of magnets and electronics.
Chemical Synthesis: Iron compounds serve as catalysts in chemical reactions, including the Haber process for ammonia synthesis.
Medicine: Iron is crucial in medical applications, particularly in treatments for iron deficiency anemia.
Food and Agriculture: Iron compounds are used as dietary supplements and in fertilizers to promote plant growth.
This article has explored the multifaceted nature of iron, delving into its various isotopes, widespread applications, essential physical and chemical properties, and the intricate process of its preparation. Iron’s significance in industrial, technological, and biological contexts underscores its irreplaceable role in modern society and highlights the ongoing need for understanding and innovating with this fundamental element.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Iron?
24
26
28
30
Which form of iron is used to make steel?
Wrought iron
Cast iron
Pig iron
Galvanized iron
Iron is essential for:
Bone formation
Blood clotting
Oxygen transport in blood
Immune function
Which type of iron ore is the highest in iron content?
Hematite
Magnetite
Limonite
Siderite
Iron deficiency in humans can lead to:
Anemia
Hyperactivity
Hypertension
Insomnia
Which industrial process is primarily used to extract iron from its ore?
Electrolysis
Fractional distillation
Smelting
Sublimation
The majority of Earth's iron is located in:
The atmosphere
The crust
The core
The oceans
What property of iron makes it particularly useful for construction?
Flexibility
Ductility
High melting point
Magnetic properties
Which alloy is formed by combining iron with carbon?
Bronze
Brass
Steel
Pewter
Iron plays a role in which environmental issue when it is released as a waste product?
Air pollution
Water pollution
Soil degradation
Noise pollution
Before you leave, take our quick quiz to enhance your learning!

