What is the atomic number of Platinum?
76
77
78
79
On a captivating journey through the world of Platinum, a symbol of luxury and a cornerstone in various scientific fields. This comprehensive guide sheds light on the definition, meaning, and multifaceted uses of Platinum, from adorning the finest jewelry to driving innovations in medicine and technology. With its unique properties and compounds, Platinum stands out as a metal of unparalleled importance. Through vivid examples, this exploration uncovers the mysteries behind Platinum’s enduring value and its pivotal role in advancing modern science and industry. Discover the versatility and elegance of Platinum, a metal that continues to shape our world and inspire endless possibilities.
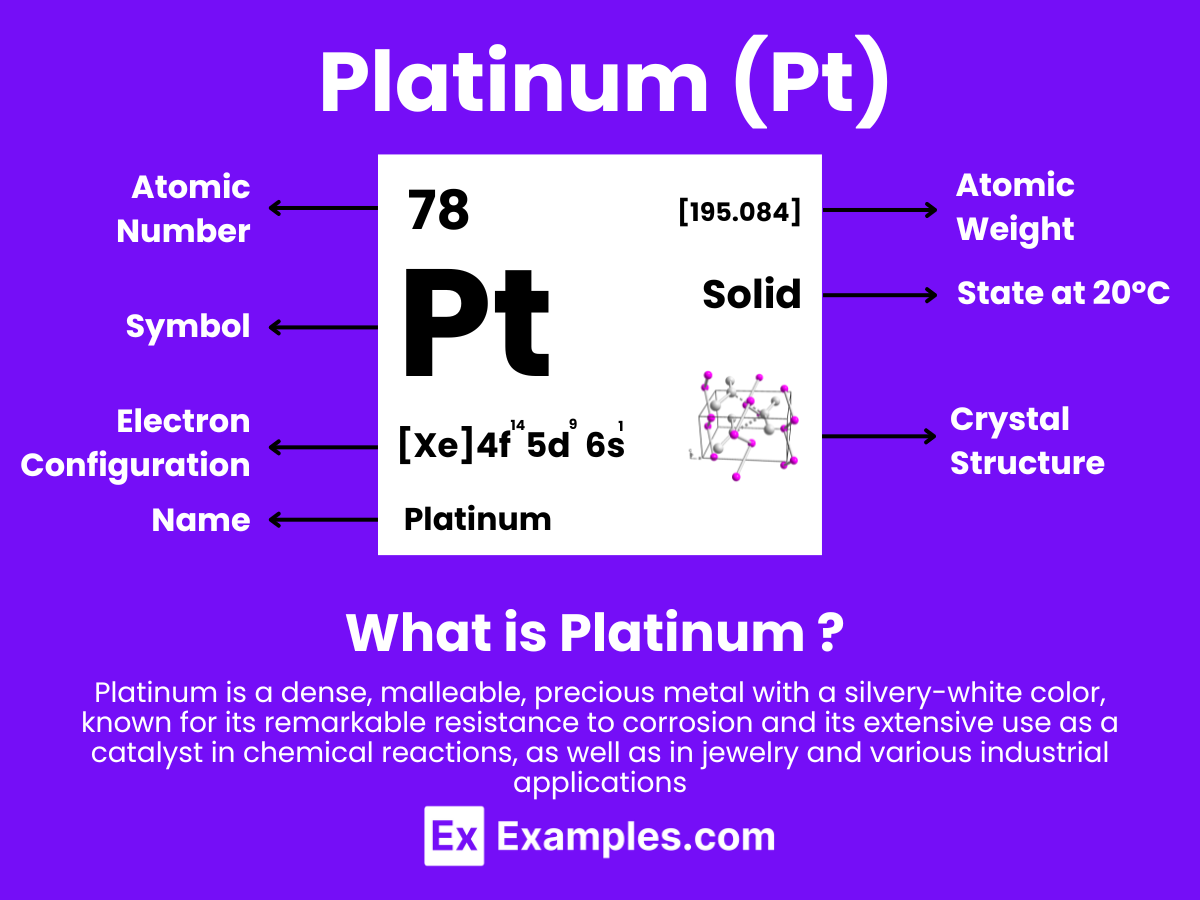
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes and is one of the rarer elements in the Earth’s crust. Platinum is known for its remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal.
Platinum, in contrast to hydrogen, is a metallic element with distinctive characteristics that enable a wide range of applications, including remarkable stability in both solid and liquid forms. The behavior of platinum at the atomic and molecular levels significantly diverges from that of hydrogen, owing to its position as a transition metal in the periodic table and its distinct metallic characteristics.
Atomic Level: Each platinum atom (Pt) contains 78 protons in its nucleus and is expected to have 78 electrons orbiting around it. The electron configuration of platinum is [Xe] 4f¹⁴ 5d⁹ 6s¹, indicating a complex electron configuration that allows for various oxidation states, similar to other elements in group 10 of the periodic table. This contributes to its significant chemical reactivity and the ability to form a variety of compounds under standard conditions.
Molecular Formation: Unlike hydrogen, which forms simple molecules like H₂ through covalent bonding, platinum does not form molecules in the same manner due to its metallic nature. In bulk form, platinum atoms are organized in a face-centered cubic lattice structure. This structure is characterized by metallic bonding, where electrons are delocalized over many platinum atoms, differing fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds. Platinum’s metallic form is stable and can be observed directly, thanks to its resistance to corrosion and high melting point, making it valuable for industrial, jewelry, and catalytic applications
| Physical Property | Description |
|---|---|
| Atomic Number | 78 |
| Atomic Mass | 195.084 u |
| Density | 21.45 g/cm³ at 20°C |
| Melting Point | 1768.3°C |
| Boiling Point | 3825°C |
| State at 20°C | Solid |
| Color | Silvery-white |
| Electrical Conductivity | Good conductor of electricity |
Platinum is a transition metal with notable chemical stability and catalytic properties. Its electron configuration is [Xe] 4f¹⁴ 5d⁹ 6s¹, which contributes to its versatility in forming compounds, primarily in the +2 and +4 oxidation states.
| Thermodynamic Property | Description |
|---|---|
| Heat of Fusion | 22.17 kJ/mol |
| Heat of Vaporization | 510 kJ/mol |
| Specific Heat Capacity | 25.86 J/(mol·K) at 25°C |
| Thermal Conductivity | 71.6 W/(m·K) |
| Thermal Expansion | 8.8 µm/(m·K) at 25°C |
| Material Property | Description |
|---|---|
| Hardness | 3.5 – 4 (Mohs scale) |
| Tensile Strength | 125 – 240 MPa |
| Malleability | High; can be beaten into thin sheets |
| Ductility | High; can be drawn into fine wire |
| Elastic Modulus | 168 GPa |
| Poisson’s Ratio | Approximately 0.38 |
| Property | Value |
|---|---|
| Electrical Conductivity | Good conductor, approximately 9.4 × 10^6 S/m |
| Magnetic Susceptibility | Paramagnetic at room temperature |
| Thermal Conductivity | 71.6 W/(m·K) at 25 °C |
| Electrical Resistivity | 10.6 nΩ·m at 20 °C |
| Reflectivity | Relatively high, especially for infrared light |
| Permeability | Slightly greater than 1 (paramagnetic) |
| Property | Value |
|---|---|
| Atomic Number | 78 |
| Atomic Mass | 195.084 u |
| Isotopes | Natural platinum consists of 5 stable isotopes: Pt-190, Pt-192, Pt-194, Pt-195, Pt-196, and Pt-198 |
| Radioisotopes | Pt-193 with a half-life of 50 years; others with shorter half-lives |
| Neutron Cross Section | Pt-195 has a high neutron absorption cross-section, important in nuclear reactors |
| Stable Isotope Natural Abundance | Varies, with Pt-195 being the most abundant at approximately 33.83% |
| Neutron Number for Stable Isotopes | Ranges from 112 to 116 for the stable isotopes |
| Fissionability | Not fissionable, but used as a neutron reflector in some nuclear reactor designs |
The preparation of platinum typically involves complex processes to extract and refine the metal from its ores. Platinum is often found naturally alloyed with small amounts of other platinum-group metals, including palladium, rhodium, osmium, iridium, and ruthenium. Here’s a general overview of how platinum is prepared:
| Isotope | Natural Abundance (%) | Half-life | Decay Mode | Notes |
|---|---|---|---|---|
| Pt-190 | 0.014 | 6.5×10^11 years | Alpha decay | Used in scientific research |
| Pt-192 | 0.782 | Stable | N/A | |
| Pt-194 | 32.967 | Stable | N/A | |
| Pt-195 | 33.832 | Stable | N/A | |
| Pt-196 | 25.242 | Stable | N/A | |
| Pt-198 | 7.163 | Stable | N/A | |
| Pt-191 | Trace | 2.96 days | Beta decay | Used in research, no commercial application |
| Pt-193 | Trace | 50 years | Beta decay | Potential use in nuclear medicine |
Platinum production involves several key steps, from mining to the final purification of the metal. The process begins with the extraction of platinum-bearing ore and ends with the production of pure platinum metal suitable for commercial use.
Platinum is a versatile metal with a wide range of applications across various industries due to its unique properties, such as excellent corrosion resistance, good thermal and electrical conductivity, and remarkable catalytic abilities.
Platinum is a highly valued, versatile element with remarkable physical and chemical properties, including its catalytic efficiency, resistance to corrosion, and electrical conductivity. It plays a critical role in various industries, from automotive to medical, and continues to be a symbol of prestige in jewelry. Understanding platinum’s isotopes further enriches our appreciation for this rare and precious metal
Text prompt
Add Tone
Chemical Compounds of Platinum
Isotopes of Platinum
Electrons
Neutrons
Protons
What is the atomic number of Platinum?
76
77
78
79
What is the chemical symbol for Platinum?
Pt
Pl
Pm
Pb
What is the primary use of Platinum in industry?
Construction
Jewelry
Agriculture
Plastic production
Which property of Platinum makes it suitable for use in catalytic converters?
High reactivity
High melting point
Good electrical conductivity
Chemical inertness
What is the melting point of Platinum?
1,546°C
1,771°C
1,964°C
2,045°C
In which form is Platinum commonly found in nature?
Oxide
Sulfide
Alloy
Native metal
Which of the following countries is the largest producer of Platinum?
Russia
South Africa
Canada
United States
Platinum is used as a catalyst in which process?
Haber process
Fischer-Tropsch synthesis
Contact process
Steam reforming
Platinum is often alloyed with which metal to make jewelry?
Silver
Gold
Copper
Palladium
What is the most common oxidation state of Platinum in its compounds?
+1
+2
+4
+3
Before you leave, take our quick quiz to enhance your learning!

