What is the atomic number of actinium?
87
88
89
70
Embark on a journey through the enigmatic world of actinium, a lesser-known member of the actinide series with a glow that captivates the curious mind. This guide unveils the mysteries of actinium, from its discovery to its applications in medicine and research. Explore the unique properties and potential uses of actinium, illustrating its significance through real-world examples. Delve into the atomic structure, preparation, and safety considerations surrounding this radioactive element, highlighting its role in advancing technology and health sciences.
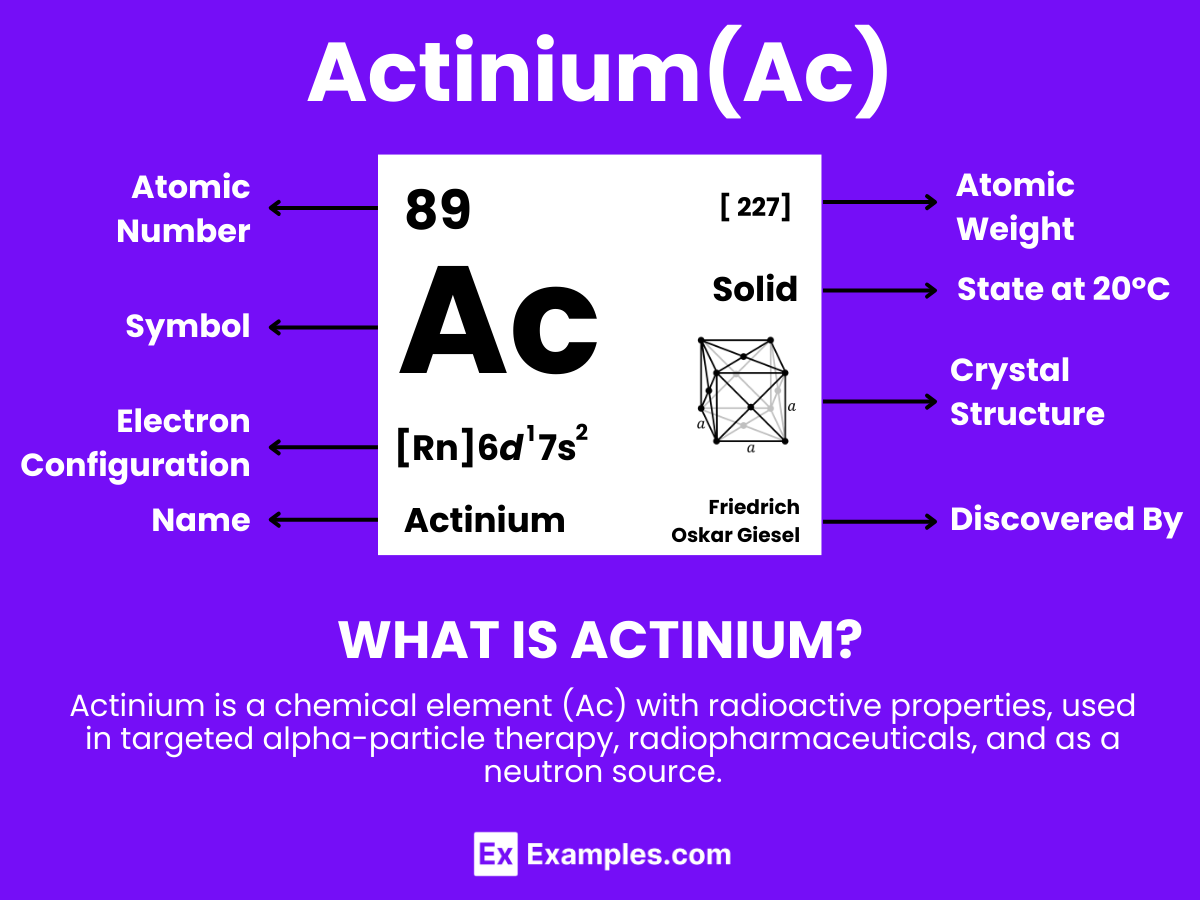
Actinium is a soft, silvery-white radioactive metallic element that showcases unique properties and specialized applications, with the atomic number 89. Actinium is known for its strong radioactivity, making it stand out among elements. It does not occur freely in nature but is found in trace amounts in uranium and thorium ores, from which it is extracted. The element plays a pivotal role in the field of nuclear science, particularly in the production of neutrons when bombarded with alpha particles, and is utilized as a neutron source. Additionally, actinium is significant in the development of cancer treatments through targeted alpha therapy (TAT), exploiting its radioactive properties to destroy cancer cells with minimal impact on surrounding healthy tissue. .
Actinium is a fascinating element with unique properties, primarily due to its atomic structure.
| Property | Value |
|---|---|
| Atomic Number | 89 |
| Atomic Mass | 227 u |
| Density | 10 g/cm³ (approx.) |
| Melting Point | 1050°C (1922°F) |
| Boiling Point | 3200°C (approx.) (5782°F) |
| State at Room Temperature | Solid |
| Color | Silvery-white, glowing with a pale blue light |
| Radioactivity | Highly Radioactive |
| Heat of Fusion | 14 kJ/mol (approx.) |
| Thermal Conductivity | 12 W/(m·K) (approx.) |
Actinium is a radioactive element known for its +3 oxidation state and reactivity with water and acids.
Actinium reacts with water to form actinium hydroxide and hydrogen gas:
Ac+3H₂O→Ac(OH)₃+32H₂
Actinium dissolves in acids like hydrochloric acid, forming actinium chloride and hydrogen:
Ac+3HCl→AcCl₃+32H₂
| Property | Value |
|---|---|
| Melting Point | 1050°C (1922°F) |
| Boiling Point | 3200°C (estimated) |
| Specific Heat Capacity | Data not readily available |
| Heat of Fusion | Data not readily available |
| Heat of Vaporization | Data not readily available |
| Thermal Conductivity | Data not readily available |
| Property | Value |
|---|---|
| State at Room Temperature | Solid |
| Density | Approx. 10 g/cm³ |
| Appearance | Silvery-white, glowing with a pale blue light |
| Molar Volume | Data not readily available |
| Hardness | Data not readily available |
| Modulus of Elasticity | Data not readily available |
| Property | Value |
|---|---|
| Natural Isotopes | Actinium-227 (most stable isotope) |
| Radioactive Decay Modes | Alpha emission primarily |
| Half-life of Actinium-227 | 21.77 years |
| Neutron Cross Section | Data not readily available |
| Neutron Mass Absorption | Data not readily available |
| Isotopic Abundance | Trace amounts, typically produced synthetically |
Actinium, a rare and highly radioactive element, is primarily produced through the neutron irradiation of radium-226 or thorium-232 in nuclear reactors. Here’s an overview of the process:
Neutron Irradiation
Production of Actinium-227
Chemical Separation
Final Form
| Isotope | Half-Life | Notes |
|---|---|---|
| Actinium-225 | 10 days | Used in targeted alpha therapy for cancer treatment. |
| Actinium-226 | 29.37 hours | Exhibits strong radioactivity, used in research. |
| Actinium-227 | 21.77 years | Acts as a parent isotope in the actinium decay series, used in thermoelectric generators. |
| Actinium-228 | 6.15 hours | Results from the decay of thorium-228, used in research. |
Actinium, due to its radioactivity and the unique properties of its isotopes, has several important applications, particularly in the fields of medicine and research:
Actinium is a rare and highly radioactive element with no stable isotopes, making its production complex and specialized.
Exploring actinium reveals a realm where science meets the challenges of handling a rare and radioactive element. Despite its complexities, actinium’s potential in medicine and research underscores its value. This journey through actinium’s properties and preparation showcases the intricate balance between its inherent risks and its promising contributions to science and healthcare, highlighting the relentless pursuit of knowledge and innovation in the atomic world
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons (89)
Neutrons (138)
Protons (89)
What is the atomic number of actinium?
87
88
89
70
Actinium is classified under which category of elements?
Alkali metals
Alkaline earth metals
Transition metals
Actinides
What is the symbol for actinium on the periodic table?
Ac
At
Am
As
Which of the following best describes actinium's appearance?
Colorless gas
Dark red liquid
Silvery metal
Yellow powder
What is the primary use of actinium-225 in medicine?
Diagnostic imaging
Radiotherapy
Pain relief
Infection control
Actinium was discovered by which scientist?
Marie Curie
Friedrich Oskar Giesel
Ernest Rutherford
Dmitri Mendeleev
What is the half-life of actinium-227?
10 years
21.8 years
50 years
100 years
In which type of ores is actinium commonly found?
Bauxite ores
Phosphate ores
Uranium ores
Iron ores
Which property makes actinium particularly dangerous to handle?
Corrosiveness
Radioactivity
Toxicity
Flammability
Actinium has similar chemical properties to which element?
Calcium
Radium
Thorium
Lead
Before you leave, take our quick quiz to enhance your learning!

