What type of bond is primarily present in ammonium nitrate?
Covalent bond
Ionic bond
Metallic bond
Hydrogen bond


Ammonium nitrate is a chemical compound that plays a crucial role in agriculture and various industrial processes. This compound, represented by the chemical formula NH₄NO₃, is primarily used as a high-nitrogen fertilizer because it efficiently provides essential nutrients to plants, helping them grow stronger and healthier. Additionally, ammonium nitrate is also a key component in the production of explosives used in mining and construction. Its ability to release a large amount of gas and energy when heated makes it extremely effective for such purposes. Despite its benefits, it is important to handle ammonium nitrate with care due to its potential for dangerous reactions under certain conditions
| Property | Value |
|---|---|
| Formula | NH₄NO₃ |
| Hill Formula | H₄N₂O₃ |
| Name | Ammonium Nitrate |
| Alternate Names | Ammonia; Nitric Acid, Ammonium Nitricum, Azane; Nitric Acid, Nitric Acid, Ammonium Salt |
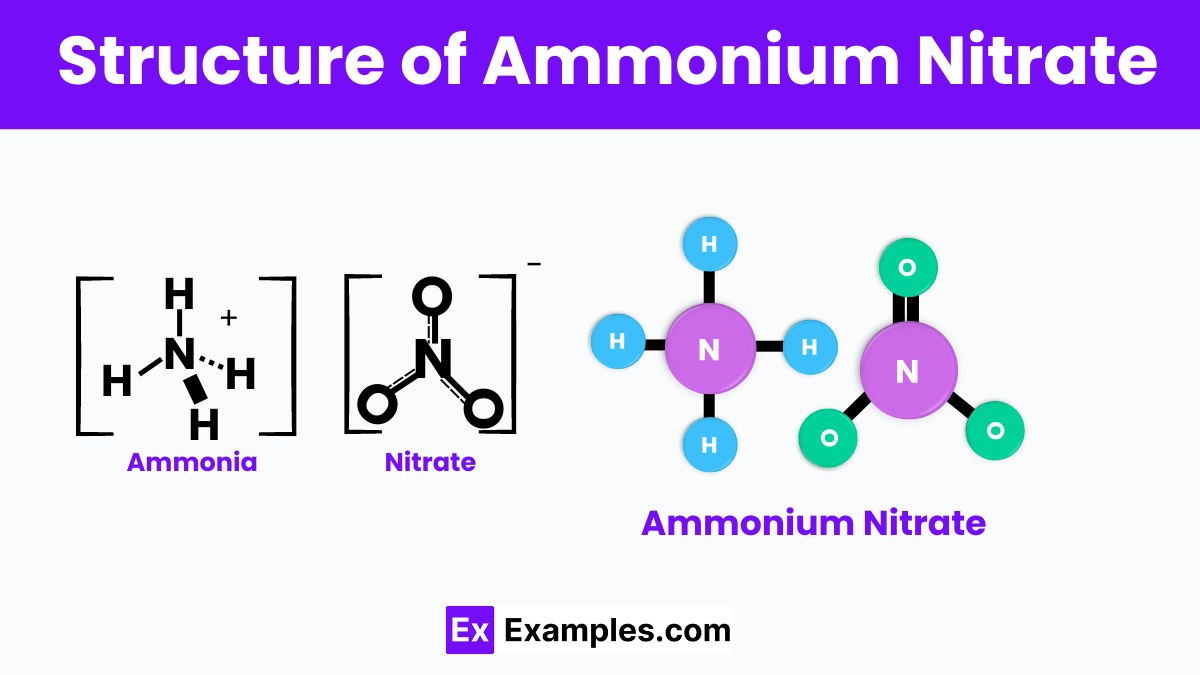
Ammonium nitrate is a chemical compound with a simple structure consisting of ammonium (NH₄⁺) and nitrate (NO₃⁻) ions. In this compound, the positively charged ammonium ion and the negatively charged nitrate ion are bonded together through ionic bonds. This structure makes ammonium nitrate highly soluble in water, allowing it to release nitrogen—an essential element for plant growth—into the soil quickly. Its crystalline form enhances its stability, making it an effective fertilizer and a component in certain explosives where rapid gas release is required.
Ammonium nitrate is prepared through a simple chemical reaction where ammonia (NH₃) reacts with nitric acid (HNO₃). The chemical equation for this reaction is:
In this process, ammonia gas is neutralized by nitric acid in a controlled environment to form ammonium nitrate, which crystallizes as a solid. To ensure the reaction is efficient and safe, it is typically conducted at lower temperatures to manage the exothermic nature of the reaction—meaning it releases heat. Once formed, the ammonium nitrate is purified and dried to form the white granules or crystals commonly used in fertilizers and explosives. This method is favored for its straightforwardness and effectiveness in producing high-quality ammonium nitrate.
| Property | Description |
|---|---|
| Appearance | White, crystalline solid that may be granulated for agricultural use. |
| Solubility | Highly soluble in water, which makes it effective for use as a quick-release fertilizer. |
| Density | 1.72 g/cm³, which is relatively dense, allowing it to sink in water. |
| Melting Point | Decomposes around 169°C (336°F); does not melt but rather breaks down, releasing gases. |
| Boiling Point | Does not have a true boiling point as it decomposes before boiling. |
| Hygroscopicity | Absorbs moisture from the air, which requires it to be stored in moisture-proof containers. |
| Property | Value |
|---|---|
| CAS Registry Number | 6484-52-2 |
| PubChem Compound ID | 22985 |
| SMILES Identifier | N+([O-])[O-].[NH4+] |
| RTECS Number | BR9050000 |
| MDL Number | MFCD00011425 |
| Property | Value |
|---|---|
| NFPA Health Rating | 0 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 3 |
| NFPA Hazards | Oxidizing agent |
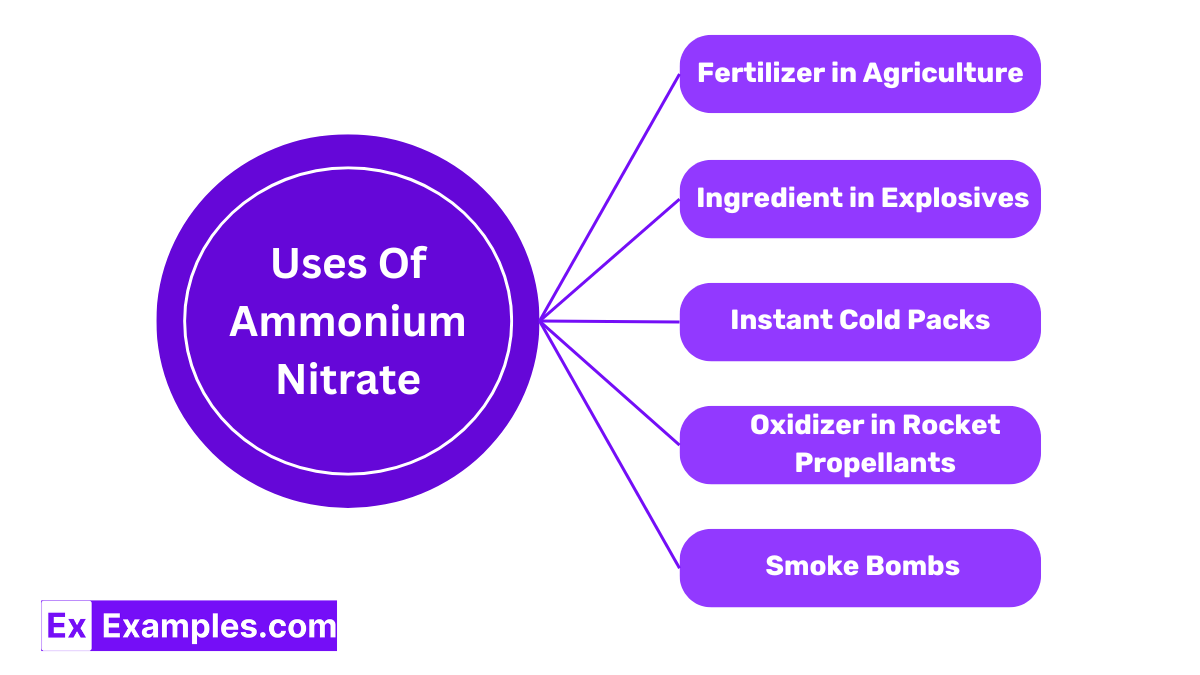
Ammonium nitrate is a popular fertilizer due to its high nitrogen content, which is essential for the healthy growth of plants. It helps increase crop yields and improves the quality of the plants.
It is a key component in many explosives used in mining, quarrying, and civil construction due to its ability to release a large amount of gases when decomposed by heat, which aids in breaking rock and other hard materials.
Ammonium nitrate is used in instant cold packs for first aid; when water dissolves the salt, it absorbs heat, thus providing an immediate cooling effect.
It is used in the production of nitrous oxide. It also known as laughing gas, which is used as an anesthetic in dentistry and surgery.
Ammonium nitrate serves as an oxidizer in rocket propellants, helping to burn the fuel in a controlled manner to propel rockets.
In the pyrotechnics industry, ammonium nitrate is used to create the oxygen needed for the combustion of various compounds in fireworks, contributing to their colorful displays.
It can be used as an antimicrobial agent in food packaging to help extend the shelf life of the packaged goods by inhibiting the growth of microorganisms.
Ammonium nitrate is also utilized in the production of smoke bombs for both entertainment and tactical purposes, where it helps produce large volumes of smoke.
Ammonium nitrate is banned in some places due to its high explosiveness and potential for misuse in improvised explosive devices.
Ammonium nitrate can be toxic, causing irritation to eyes, skin, and respiratory system upon direct exposure.
Yes, ammonium nitrate is still widely used as a fertilizer and a component in explosives for industrial use.
Ammonium nitrate is highly explosive because it acts as a strong oxidizer, rapidly releasing gases that expand violently when heated.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What type of bond is primarily present in ammonium nitrate?
Covalent bond
Ionic bond
Metallic bond
Hydrogen bond
Which element is present in ammonium nitrate but not in pure carbon?
Nitrogen
Hydrogen
Oxygen
All of the above
What is the primary use of ammonium nitrate?
As a pesticide
As a fertilizer
As a food preservative
As a fuel additive
Ammonium nitrate is also commonly used in which application besides agriculture?
Fireworks
Explosives
Pharmaceuticals
Water treatment
Which safety concern is associated with ammonium nitrate?
Toxicity
Flammability
Explosiveness
Corrosiveness
What is the molar mass of ammonium nitrate?
80 g/mol
96 g/mol
108 g/mol
124 g/mol
In the presence of a reducing agent, ammonium nitrate can decompose to form:
Ammonia and water
Nitrogen gas and water
Oxygen and hydrogen gas
Carbon dioxide and nitrogen gas
What is the primary environmental concern related to the use of ammonium nitrate fertilizers?
Soil erosion
Air pollution
Water contamination
Noise pollution
Ammonium nitrate is classified under which category of chemicals?
Reducing agents
Bases
Acids
Oxidizing agents
Which process is used to produce ammonium nitrate industrially?
Haber process
Ostwald process
Neutralization reaction
Hydrolysis reaction
Before you leave, take our quick quiz to enhance your learning!

